推薦產品
品質等級
產品線
Novabiochem®
化驗
≥90.0% (acidimetric)
≥95.0% (HPLC)
≥97% (TLC)
形狀
powder
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
製造商/商標名
Novabiochem®
應用
peptide synthesis
官能基
Fmoc
儲存溫度
−20°C (−15°C to −25°C)
InChI
1S/C25H24NO8P/c27-24(28)23(16-34-35(30,31)33-14-17-8-2-1-3-9-17)26-25(29)32-15-22-20-12-6-4-10-18(20)19-11-5-7-13-21(19)22/h1-13,22-23H,14-16H2,(H,26,29)(H,27,28)(H,30,31)/t23-/m0/s1
InChI 密鑰
ZBPUWGDUVAAWJY-QHCPKHFHSA-N
一般說明
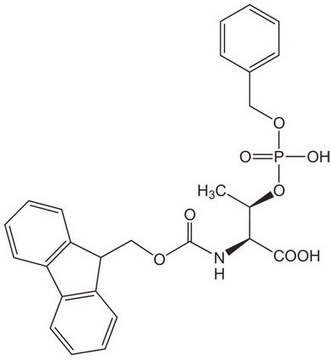
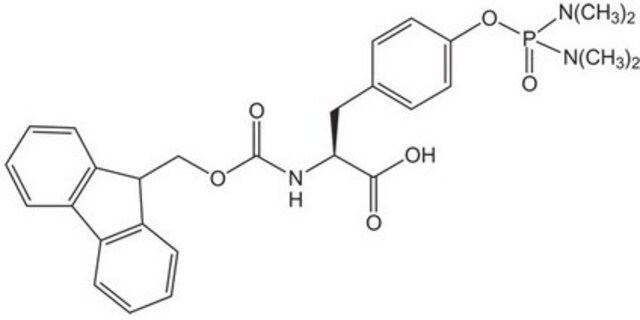
An excellent building block for the preparation of phosphoserine-containing peptides [1] by Fmoc SPPS. This derivative can be introduced using standard activation methods, such as PyBOP® and TBTU. The monoprotected phosphoserine residue once incorporated is stable to piperidine. Using this reagent, even peptides containing multiple phosphorylation sites can be prepared efficiently by standard Fmoc SPPS methods [2]. Applications of this derivative include the preparation of phospholamban [3], a 52 residue peptide containing both phosphoserine and phosphothreonine, and human salivary statherin, a 42 residue phosphoserine peptide [4]; for other examples see references [5,6,7,8].Recently, β-piperidinylalanine formation has been shown to occur during Fmoc deprotection of N-terminal Ser(PO(OBzl)OH), particularly under microwave conditions. This side reaction can be eliminated by using cyclohexylamine or DBU just for this Fmoc deprotection step [9].
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] T. Wakamiya, et al. (1994) Chem. Lett., 1099.
[2] P. White & J. Beythien in ′Innovations & Perspectives in Solid Phase Synthesis and Combinatorial Libraries, 4th International Symposium′, Mayflower Scientific Ltd., Birmingham, 1996, pp. 557.
[3] H. Schmid, et al., Poster 423 presented at the 15th American Peptide Symposium, Nashville, 1997.
[4] T. L. Gururaja & M. J. Levine (1996) Pept. Res., 9, 283.
[5] T. Vorherr, et al. (1995) Bioorg. Med. Chem. Lett., 5, 2661.
[6] G. Shapiro, et al. (1996) Bioorg. Med. Chem. Lett., 6, 409.
[7] M. John, et al. (1996) Pept. Res., 9, 71.
[8] K. Teruya, et al. (2004) J. Pept. Sci., 10, 479.
[9] T. J. Attard, et al. (2009) Int. J. Pept. Res. Ther., 15, 69.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] T. Wakamiya, et al. (1994) Chem. Lett., 1099.
[2] P. White & J. Beythien in ′Innovations & Perspectives in Solid Phase Synthesis and Combinatorial Libraries, 4th International Symposium′, Mayflower Scientific Ltd., Birmingham, 1996, pp. 557.
[3] H. Schmid, et al., Poster 423 presented at the 15th American Peptide Symposium, Nashville, 1997.
[4] T. L. Gururaja & M. J. Levine (1996) Pept. Res., 9, 283.
[5] T. Vorherr, et al. (1995) Bioorg. Med. Chem. Lett., 5, 2661.
[6] G. Shapiro, et al. (1996) Bioorg. Med. Chem. Lett., 6, 409.
[7] M. John, et al. (1996) Pept. Res., 9, 71.
[8] K. Teruya, et al. (2004) J. Pept. Sci., 10, 479.
[9] T. J. Attard, et al. (2009) Int. J. Pept. Res. Ther., 15, 69.
聯結
Replaces: 04-12-1154
分析報告
Colour (visual): white to slight yellow to beige
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.0 % (a/a)
Purity (TLC(CMA1)): ≥ 97 %
Assay (HPLC, area%): ≥ 95.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Assay (acidimetric): ≥ 90.0 %
Water (K. F.): ≤ 1.0 %
Ethyl acetate (HS-GC): ≤ 0.5 %
Acetate (IC): ≤ 0.1 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.0 % (a/a)
Purity (TLC(CMA1)): ≥ 97 %
Assay (HPLC, area%): ≥ 95.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Assay (acidimetric): ≥ 90.0 %
Water (K. F.): ≤ 1.0 %
Ethyl acetate (HS-GC): ≤ 0.5 %
Acetate (IC): ≤ 0.1 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
法律資訊
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
PyBOP is a registered trademark of Merck KGaA, Darmstadt, Germany
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務