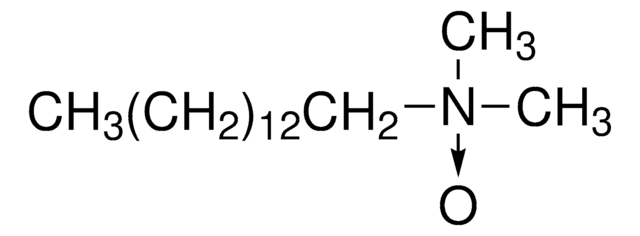推薦產品
品質等級
產品線
EMPROVE® EVOLVE
形狀
liquid
環保替代產品特色
Designing Safer Chemicals
Use of Renewable Feedstocks
Design for Degradation
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
應用
pharma/biopharma processes
環保替代類別
儲存溫度
15-25°C
SMILES 字串
[N](=O)(CCCCCCCCCCCCCC)(C)C
InChI
1S/C16H35NO/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17(2,3)18/h4-16H2,1-3H3
InChI 密鑰
ONHFWHCMZAJCFB-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Detergents, such as non-ionic surfactants, are used in biomanufacturing processes for a variety of applications including viral inactivation and cell lysis. Detergent-mediated viral inactivation is widely used in multiple biotherapeutic production processes to ensure meeting regulatory requirements for viral safety.
Our SAFC portfolio of high-quality raw materials for use in biopharmaceutical processing withstands strict quality control procedures plus the documentation and expertise to help our customers meet requirements as defined by the M-Clarity Program.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of downstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Ranging from non-GMP grades for low-risk application, to IPEC-PQG GMP for higher-risk applications, we have products covering all your manufacturing needs.
We offer a range of high quality critical raw materials plus the documentation and expertise to help our customers meet stringent regulatory requirements. Our SAFC portfolio of high-quality products withstands strict quality control procedures.
Our SAFC portfolio of high-quality raw materials for use in biopharmaceutical processing withstands strict quality control procedures plus the documentation and expertise to help our customers meet requirements as defined by the M-Clarity Program.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of downstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Ranging from non-GMP grades for low-risk application, to IPEC-PQG GMP for higher-risk applications, we have products covering all your manufacturing needs.
We offer a range of high quality critical raw materials plus the documentation and expertise to help our customers meet stringent regulatory requirements. Our SAFC portfolio of high-quality products withstands strict quality control procedures.
應用
REACH compliantdetergent used in viral inactivation or cell lysis.
法律資訊
DEVIRON is a registered trademark of Merck KGaA, Darmstadt, Germany
Emprove is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Irrit. 2
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
212.0 °F
閃點(°C)
> 100 °C
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
相關內容
Learn more on mAb downstream processing, more specifically virus inactivation and the relevant associated products.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務