全部照片(3)
About This Item
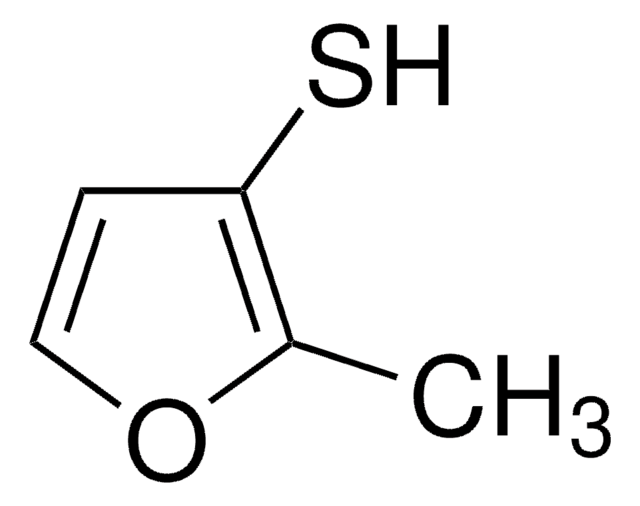
經驗公式(希爾表示法):
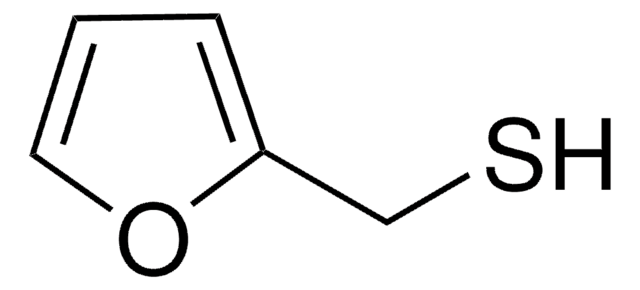
C5H6OS
CAS號碼:
分子量::
114.17
FEMA號碼:
2493
Beilstein:
383594
EC號碼:
歐洲委員會號碼:
2202
MDL號碼:
分類程式碼代碼:
12164502
PubChem物質ID:
Flavis號碼:
13.026
NACRES:
NA.21
agency:
follows IFRA guidelines
meets purity specifications of JECFA
meets purity specifications of JECFA
推薦產品
等級
FG
Fragrance grade
Halal
Kosher
natural
品質等級
agency
follows IFRA guidelines
meets purity specifications of JECFA
法律遵循
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
化驗
98%
環保替代產品特色
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
折射率
n20/D 1.531 (lit.)
bp
155 °C (lit.)
密度
1.132 g/mL at 25 °C (lit.)
應用
flavors and fragrances
文件
see Safety & Documentation for available documents
食物過敏原
no known allergens
香料過敏原
no known allergens
環保替代類別
感官的
coffee; meaty; roasted; sulfurous
SMILES 字串
SCc1ccco1
InChI
1S/C5H6OS/c7-4-5-2-1-3-6-5/h1-3,7H,4H2
InChI 密鑰
ZFFTZDQKIXPDAF-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
We are committed to bringing you greener alternative products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product is Biobased and thus aligns with "Less Hazardous Chemical Syntheses" and "Use of Renewable Feedstocks".
其他說明
Natural occurrence: Burley tobacco, bread, cocoa, coffee, juniper berry oil, potato chips, roasted barley, whiskey.
客戶也查看了
Christoph Müller et al.
Journal of agricultural and food chemistry, 54(26), 10076-10085 (2006-12-21)
Recent investigations demonstrated that the reaction of odor-active thiols such as 2-furfurylthiol with thermally generated chlorogenic acid degradation products is responsible for the rapid aroma staling of coffee beverages. To get a clear understanding of the molecular mechanisms underlying this
Christoph Müller et al.
Journal of agricultural and food chemistry, 55(10), 4095-4102 (2007-04-19)
To gain a more comprehensive knowledge of the contribution of recently identified phenol/thiol conjugates to the storage-induced degradation of odorous thiols, the concentrations of the sulfury-roasty smelling key odorant 2-furfurylthiol and the concentrations of the putative thiol-receptive di- and trihydroxybenzenes
Christoph Müller et al.
Journal of agricultural and food chemistry, 53(7), 2623-2629 (2005-03-31)
The purpose of the following study was to investigate the influence of coffee roasting on the thiol-binding activity of coffee beverages, and to investigate the potential of various green bean compounds as precursors of thiol-binding sites by using promising "in
Brian G Lake et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 41(12), 1761-1770 (2003-10-18)
The metabolism of two thiofurans, namely furfuryl mercaptan (FM) and 2-methyl-3-furanthiol (MTF), to their corresponding methyl sulphide and methyl sulphoxide derivatives has been studied in male Sprague-Dawley rat hepatocytes and liver microsomes. Rat hepatocytes converted FM to furfuryl methyl sulphoxide
Luigi Poisson et al.
Journal of agricultural and food chemistry, 57(21), 9923-9931 (2009-10-13)
The formation of several key odorants, such as 2-furfurylthiol (FFT), alkylpyrazines, and diketones, was studied upon coffee roasting. The approach involved the incorporation of potential precursors in green coffee beans by means of biomimetic in-bean and spiking experiments. Both labeled
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| W249311-100G-K | 4061837876936 |
| W249311-SAMPLE-K | 4061837515156 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務