推薦產品
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.481 (lit.)
bp
64-65 °C/19 mmHg (lit.)
密度
0.98 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
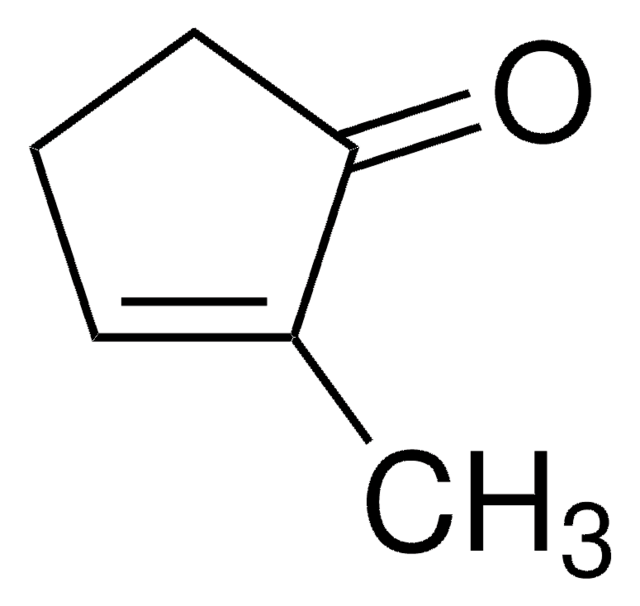
O=C1CCC=C1
InChI
1S/C5H6O/c6-5-3-1-2-4-5/h1,3H,2,4H2
InChI 密鑰
BZKFMUIJRXWWQK-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
用于许多加成反应的多功能亲电试剂,包括有机铜亲核试剂的共轭加成、与硅烯醇醚和硅氧烷的 Michael 加成、Diels-Alder 环加成以及磷鎓基硅烷化。
客戶也查看了
Yoshihiro Sumiyoshi et al.
The Journal of chemical physics, 125(12), 124307-124307 (2006-10-04)
Pure rotational transitions in the ground state for Ar-OH and Ar-OD [Y. Ohshima et al., J. Chem. Phys. 95, 7001 (1991) and Y. Endo et al., Faraday Discuss. 97, 341 (1994)], those in the excited states of the OH vibration
Marc Revés et al.
Organic letters, 14(13), 3534-3537 (2012-06-28)
1,2,3,4-Tetramethyl-bicyclo[2.2.1]hepta-2,5-diene (TMNBD, for tetramethylnorbornadiene) has been prepared and used successfully as an acetylene equivalent in the synthesis of substituted cyclopentenones. TMNBD is easily accessible on a multigram scale and displays excellent reactivity toward the intermolecular Pauson-Khand reaction. Conjugate additions on
Filippo De Simone et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(51), 14527-14538 (2011-11-25)
The Nazarov cyclization of divinyl ketones gives access to cyclopentenones. Replacing one of the vinyl groups by a cyclopropane leads to a formal homo-Nazarov process for the synthesis of cyclohexenones. In contrast to the Nazarov reaction, the cyclization of vinyl-cyclopropyl
Xiaoxun Li et al.
Organic letters, 14(6), 1584-1587 (2012-03-03)
Functionalized cyclopentenones were synthesized by a Rh-catalyzed carbonylation of 3-acyloxy-1,4-enynes, derived from alkynes and α,β-unsaturated aldehydes. The reaction involved a Saucy-Marbet 1,3-acyloxy migration of propargyl esters and a [4 + 1] cycloaddition of the resulting acyloxy substituted vinylallene with CO.
Daniel J Kerr et al.
Organic letters, 14(7), 1732-1735 (2012-03-30)
Oxazolidinones are powerful promoters of the Nazarov reaction, enabling the cyclization of conventionally resistant substrates to be achieved under mild conditions. They exert excellent regio- and torquoselective control in both the conventional Nazarov reaction giving cyclopentenones and in the "interrupted"
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務