推薦產品
化驗
98%
折射率
n20/D 1.494 (lit.)
bp
199-200 °C (lit.)
密度
0.971 g/mL at 25 °C (lit.)
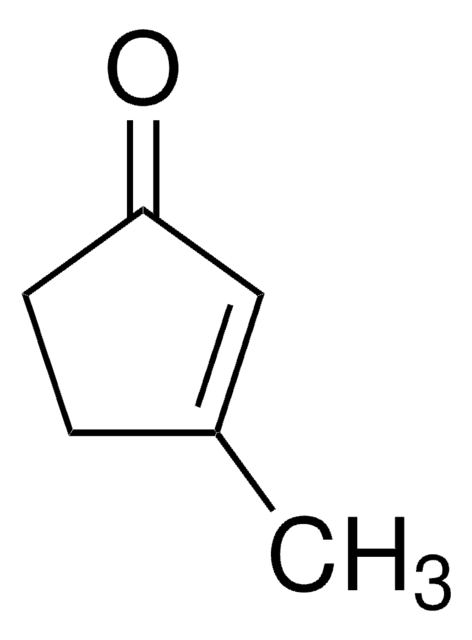
SMILES 字串
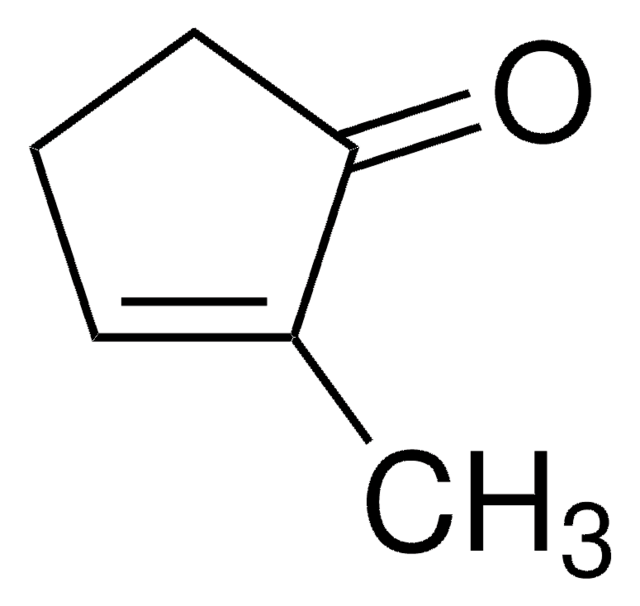
CC1=CC(=O)CCC1
InChI
1S/C7H10O/c1-6-3-2-4-7(8)5-6/h5H,2-4H2,1H3
InChI 密鑰
IITQJMYAYSNIMI-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
3-Methyl-2-cyclohexenone is an insect sex pheromone of the Douglas-fir beetle. It can be used as a starting material:
- In the total synthesis of (−)-ar-tenuifolene, a naturally occurring aromatic sesquiterpene.
- To synthesize an organic building block 2-trimethylsilyl-3-methyl-cyclohexenone.
- In the total synthesis of natural diterpenoids (+)-taiwaniaquinone H and (+)-dichroanone.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
154.4 °F - closed cup
閃點(°C)
68 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Catalytic asymmetric formal total synthesis of (+)-dichroanone and (+)-taiwaniaquinone H
Li L-Q, et al.
Tetrahedron Letters, 55(43), 5960-5962 (2014)
Catalytic enantioselective total synthesis of (−)-ar-Tenuifolene
Shaw K, et al.
Tetrahedron Letters, 61(20), 151850-151850 (2020)
Aldrichimica Acta, 16, 41-41 (1983)
Shoichiro Horita et al.
Chembiochem : a European journal of chemical biology, 16(3), 440-445 (2015-02-03)
(4R,6R)-Actinol can be stereo-selectively synthesized from ketoisophorone by a two-step conversion using a mixture of two enzymes: Candida macedoniensis old yellow enzyme (CmOYE) and Corynebacterium aquaticum (6R)-levodione reductase. However, (4S)-phorenol, an intermediate, accumulates because of the limited substrate range of
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務