推薦產品
品質等級
化驗
≥95%
形狀
liquid
折射率
n/D 1.454
密度
1.062 g/mL
儲存溫度
−20°C
SMILES 字串
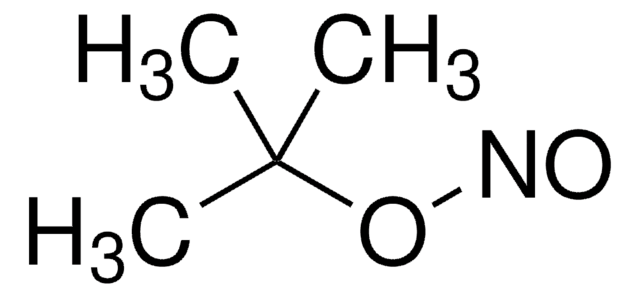
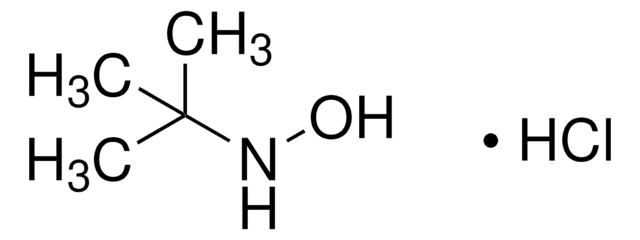
[S](=NOC(C)(C)C)=O
InChI
1S/C4H9NO2S/c1-4(2,3)7-5-8-6/h1-3H3
InChI 密鑰
QNDHWAYLAJVRCB-UHFFFAOYSA-N
應用
t-BuONSO is a stable reagent active for the synthesis of primary sulfonamides when used in conjunction with organolithium or Grignard reagents. t-BuONSO is tolerant of a variety of alkyl and aryl substrates and can be used in the synthesis of many medicinally relevant compounds.
訊號詞
Danger
危險聲明
危險分類
Resp. Sens. 1 - Self-react. C - Skin Sens. 1
儲存類別代碼
5.2 - Organic peroxides and self-reacting hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Thomas Q Davies et al.
Journal of the American Chemical Society, 142(36), 15445-15453 (2020-08-26)
Sulfoximines and sulfonimidamides are promising compounds for medicinal and agrochemistry. As monoaza analogues of sulfones and sulfonamides, respectively, they combine good physicochemical properties, high stability, and the ability to build complexity from a three-dimensional core. However, a lack of quick
Thomas Q Davies et al.
Organic letters, 22(24), 9495-9499 (2020-11-26)
Sulfonamides have played a defining role in the history of drug development and continue to be prevalent today. In particular, primary sulfonamides are common in marketed drugs. Here we describe the direct synthesis of these valuable compounds from organometallic reagents
Ze-Xin Zhang et al.
Journal of the American Chemical Society, 141(33), 13022-13027 (2019-08-10)
Sulfondiimines-the double aza-analogues of sulfones-hold significant potential as leads in discovery chemistry, yet their application in this arena has been held back by the scarcity of appropriate synthetic routes. Existing methods employ sulfides as substrates, and rely on consecutive imination
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務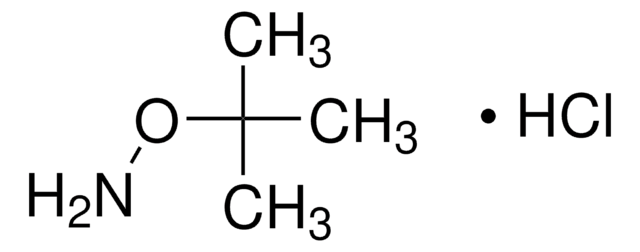

![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![1,4-二氮杂二环[2.2.2]辛烷二(二氧化硫)加合物 ≥95% (sulfur, elemental analysis)](/deepweb/assets/sigmaaldrich/product/structures/158/739/a9df497b-883d-40f1-ac45-bf699dcee9f9/640/a9df497b-883d-40f1-ac45-bf699dcee9f9.png)

