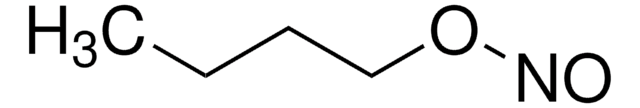
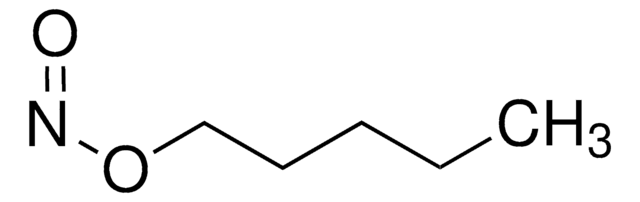
推薦產品
品質等級
化驗
90%
折射率
n20/D 1.368 (lit.)
bp
61-63 °C (lit.)
溶解度
alcohol: very soluble(lit.)
carbon disulfide: very soluble(lit.)
chloroform: very soluble(lit.)
diethyl ether: very soluble(lit.)
glycerol: insoluble (practically)(lit.)
water: slightly soluble(lit.)
密度
0.867 g/mL at 25 °C (lit.)
官能基
O-nitroso
nitroso
儲存溫度
2-8°C
SMILES 字串
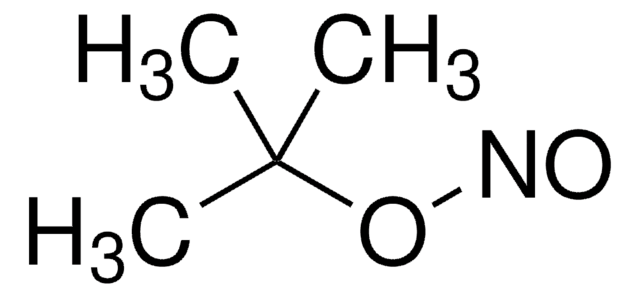
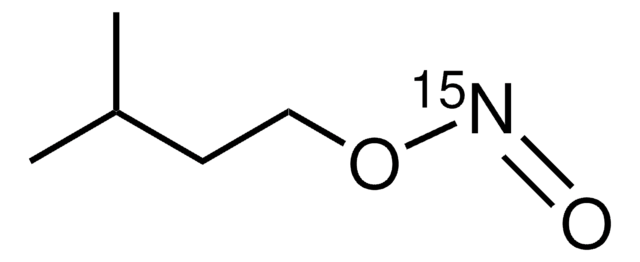
CC(C)(C)ON=O
InChI
1S/C4H9NO2/c1-4(2,3)7-5-6/h1-3H3
InChI 密鑰
IOGXOCVLYRDXLW-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
叔-亚硝酸丁酯(TBN)是一种有效的NO源。TBN参与将芳基和杂芳基胺通过光催化转化为硒化物。它还参与脂族烯烃的自由基多官能化反应。
應用
叔-亚硝酸丁酯已作为试剂:
- 用于醇、硫醇、胺和环烷烃的重氮化和亚硝化
- 用于使用芳基胺制备芳基叠氮化物
其他說明
残留物为 2-甲基-2-丙醇
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
14.0 °F - closed cup
閃點(°C)
-10 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves
客戶也查看了
Daisuke Hirose et al.
Beilstein journal of organic chemistry, 9, 1713-1717 (2013-09-26)
Water induces a change in the product of radical multifunctionalization reactions of aliphatic alkenes involving an sp(3) C-H functionalization by an 1,5-hydrogen shift using tert-butyl nitrite and molecular oxygen. The reaction without water, reported previously, gives nitrated γ-lactols, whereas the
tert-Butyl Nitrite.
Liu Y.
Synlett, 2011(08), 1184-1185 (2011)
Karine Barral et al.
Organic letters, 9(9), 1809-1811 (2007-03-30)
[reaction: see text] An efficient and improved procedure for the preparation of aromatic azides and their application in the Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition ("click reaction") is described. The synthesis of aromatic azides from the corresponding amines is accomplished under mild
Debasish Kundu et al.
Organic letters, 16(6), 1814-1817 (2014-03-14)
A novel strategy for the direct conversion of aryl- and heteroarylamines to selenides has been developed via diazotization of amines with tert-butyl nitrite in neutral medium followed by reaction with diaryl/diheteroaryl/dialkyl diselenides in one pot under photocatalysis at room temperature
The Journal of Organic Chemistry, 42, 2431-2431 (1977)
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 235385-100ML | 4061838785039 |
| 235385-25ML | 4061838785046 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務