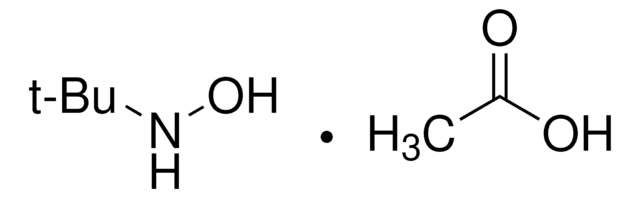推薦產品
品質等級
化驗
≥99.0% (AT)
形狀
solid
mp
~155 °C (dec.)
溶解度
H2O: soluble 0.5 g/10 mL
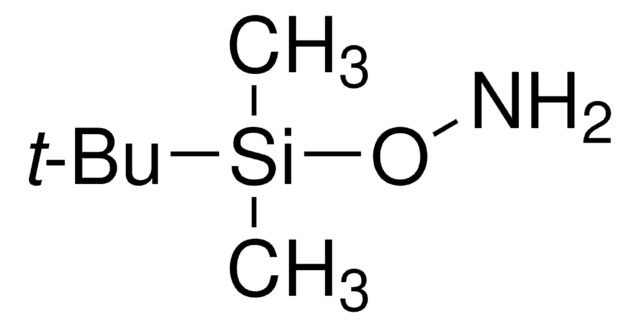
SMILES 字串
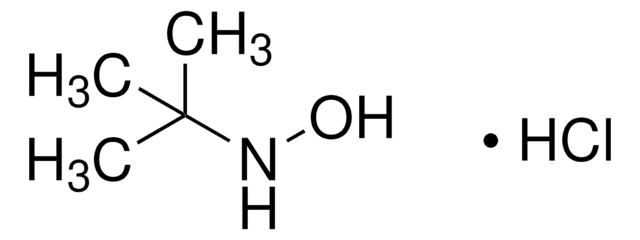
Cl.CC(C)(C)ON
InChI
1S/C4H11NO.ClH/c1-4(2,3)6-5;/h5H2,1-3H3;1H
InChI 密鑰
ZBDXGNXNXXPKJI-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
O-tert-Butylhydroxylamine hydrochloride was used in the synthesis of N-methyl-O-tert-butylhydroxylamine hydrochloride. It was also used in the preparation of N-tert-butoxyamino acids as substrates for the unambiguous synthesis of N-hydroxy peptides.
Reactant involved in synthesis of biologically active molecules including:
Reactant involved in:
- CGS 25966 derivatives for use as MMP inhibitors
- Imidazolidinedione derivatives for use as antimalarial treatments
- Pyrimidine ribonucleotide analogs as P2Y6 receptor agonists
- Rab proteins for isoprenylation and geranylgeranylation inhibition
Reactant involved in:
- Synthesis of N-(arylethyl)-O-tert-butylhydroxamates for use as Weinreb amide equivalents
- Double allylic alkylation of indole-2-hydroxamates
- SN2 substitution reactions at amide nitrogens
- Photocycloaddition to C=N bonds for synthesis of 1,3-diazepines
客戶也查看了
Klaus Kopka et al.
Nuclear medicine and biology, 31(2), 257-267 (2004-03-12)
Non-invasive measurement of matrix metalloproteinase (MMP) activity in vivo is a clinical challenge in many disease processes such as inflammation, tumor metastasis and atherosclerosis. Therefore, radioiodinated analogues of the non-peptidyl broad-spectrum MMP inhibitor (MMPI) CGS 27023A 1a were synthesized for
T. Kolasa et al.
Tetrahedron, 30, 3591-3591 (1974)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務