推薦產品
化驗
≥95%
形狀
powder or crystals
mp
144-160 °C
SMILES 字串
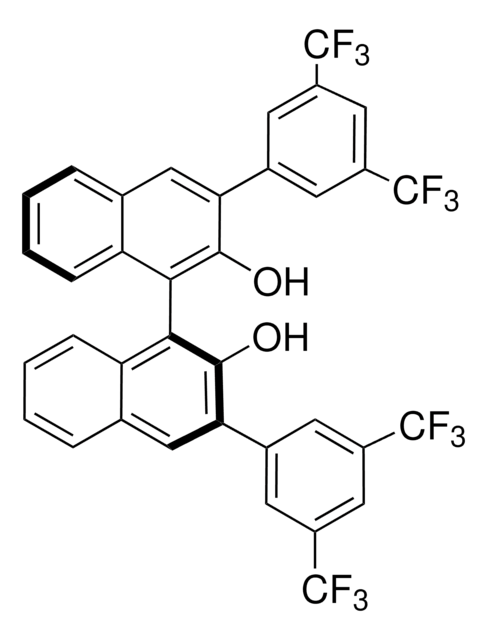
FC(F)(F)c1cc(cc(c1)C(F)(F)F)NC(=O)N(C(C)C)c2c(c6c(cc2)cccc6)c3c4c(ccc3NC(=O)Nc5cc(cc(c5)C(F)(F)F)C(F)(F)F)cccc4
InChI
1S/C41H28F12N4O2/c1-21(2)57(37(59)55-29-19-26(40(48,49)50)16-27(20-29)41(51,52)53)33-14-12-23-8-4-6-10-31(23)35(33)34-30-9-5-3-7-22(30)11-13-32(34)56-36(58)54-28-17-24(38(42,43)44)15-25(18-28)39(45,46)47/h3-21H,1-2H3,(H,55,59)(H2,54,56,58)
應用
((S)-3-(3,5-Bis(trifluoromethyl)phenyl)-1-(2′-(3-(3,5-bis(trifluoromethyl)phenyl)ureido)-[1,1′-binaphthalen]-2-yl)-1-isopropylurea is a hydrogen bonding phase-transfer catalyst capable of activating CsF for the asymmetric nucleophilic fluorination of sulfoniums.1
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Gabriele Pupo et al.
Science (New York, N.Y.), 360(6389), 638-642 (2018-05-12)
Common anionic nucleophiles such as those derived from inorganic salts have not been used for enantioselective catalysis because of their insolubility. Here, we report that merging hydrogen bonding and phase-transfer catalysis provides an effective mode of activation for nucleophiles that
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![(R)-N-[(1R,2R)-2-(3-(3,5-双(三氟甲基)苯基)脲基)环己基]-叔丁基亚磺酰胺 96%](/deepweb/assets/sigmaaldrich/product/structures/389/070/18847164-c6a7-4b4e-abcb-2dbc22493a2d/640/18847164-c6a7-4b4e-abcb-2dbc22493a2d.png)
![1-[3,5-bis(trifluoromethyl)phenyl]-3-[(1R,2R)-(-)-2-(dimethylamino)cyclohexyl]thiourea AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/236/021/d944889d-2233-4700-9f2c-caa3652d0124/640/d944889d-2233-4700-9f2c-caa3652d0124.png)
![N-[(1R,2R)-2-(1-哌啶基)环己基]-N′-[4-(三氟甲基)苯基]四酰胺 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)
![(S)-2-[[3,5-双(三氟甲基)苯基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺 97%](/deepweb/assets/sigmaaldrich/product/structures/373/888/118b46f2-6c2e-4a87-8266-c4dbcd5db51f/640/118b46f2-6c2e-4a87-8266-c4dbcd5db51f.png)
![聚[(9,9-二正辛基芴基-2,7-二基)-alt-(苯并[2,1,3]噻二唑-4,8-二基)] average Mn ≤25000](/deepweb/assets/sigmaaldrich/product/structures/428/661/1c4ebb98-9d51-48c0-96c7-e556ca425aa4/640/1c4ebb98-9d51-48c0-96c7-e556ca425aa4.png)
![1,3-双[3,5-双(三氟甲基)苯基]硫脲 95%](/deepweb/assets/sigmaaldrich/product/structures/191/427/0218c99c-65b9-4963-938c-c47a5790dfc5/640/0218c99c-65b9-4963-938c-c47a5790dfc5.png)