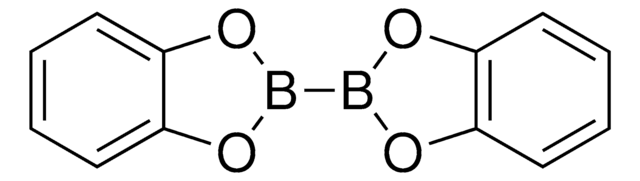
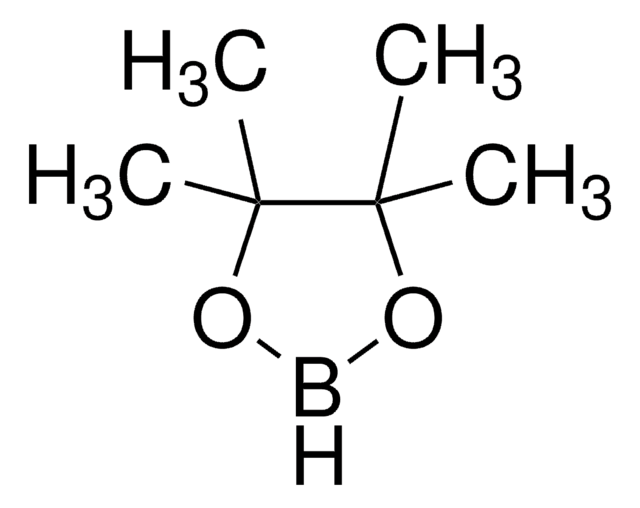
推薦產品
形狀
solid
品質等級
環保替代產品特色
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
48 °C
環保替代類別
儲存溫度
2-8°C
SMILES 字串
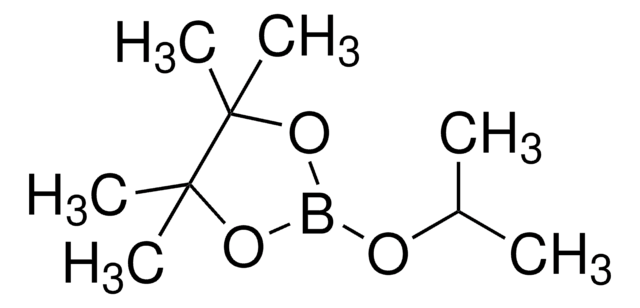
CC(C(C)(C)O1)(C)OB1CB2OC(C)(C)C(C)(C)O2
InChI
1S/C13H26B2O4/c1-10(2)11(3,4)17-14(16-10)9-15-18-12(5,6)13(7,8)19-15/h9H2,1-8H3
InChI 密鑰
MQYZGGWWHUGYDR-UHFFFAOYSA-N
相關類別
一般說明
Bis[(pinacolato)boryl]methane is an aryl boronate ester.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information.
應用
Bis-boronate has been reported by Morken and coworkers to be a key building block in the synthesis of enantioenriched secondary boronate esters, which undergo facile Suzuki-Miyaura coupling with minimal erosion of enantiopurity.
Bis[(pinacolato)boryl]methane may be used in the preparation of trans-vinyl boronate esters, via the Boron-Wittig reaction.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
客戶也查看了
A Catalytic Enantiotopic-Group-Selective Suzuki Reaction for the Construction of Chiral Organoboronates
Sun C, et al.
Journal of the American Chemical Society, 136, 6534-6537 (2014)
John R Coombs et al.
Organic letters, 17(7), 1708-1711 (2015-03-24)
A highly stereoselective boron-Wittig reaction between stable and readily accessible 1,1-bis(pinacolboronates) and aldehydes furnishes a variety of synthetically useful di- and trisubstituted vinyl boronate esters.
文章
Enantioselective alkene diboration is a valuable strategy for transforming unsaturated hydrocarbons into useful chiral building blocks.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)