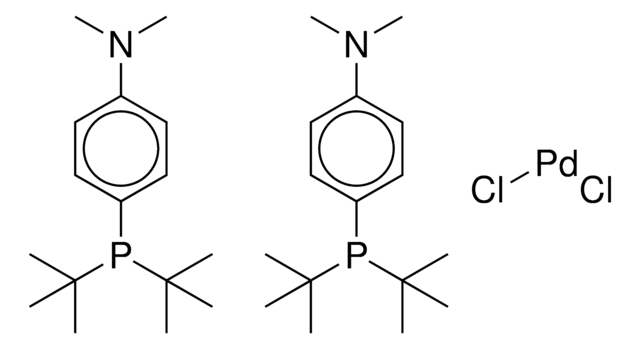
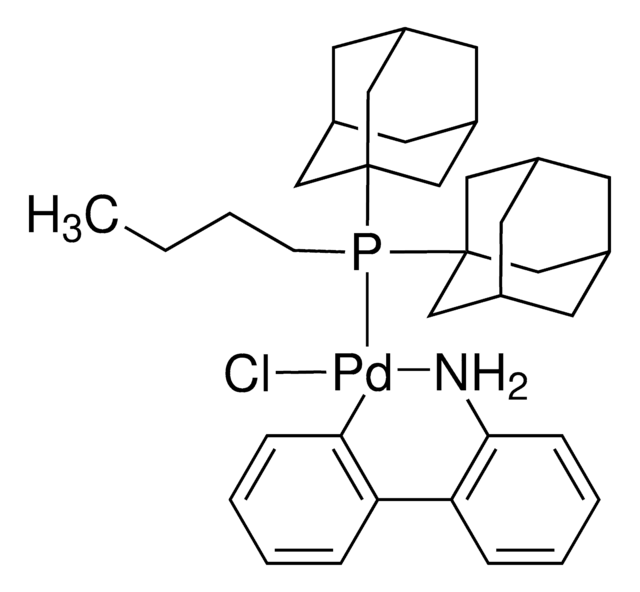
推薦產品
品質等級
化驗
98%
形狀
solid
特點
generation 2
反應適用性
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
202-210 °C
官能基
phosphine
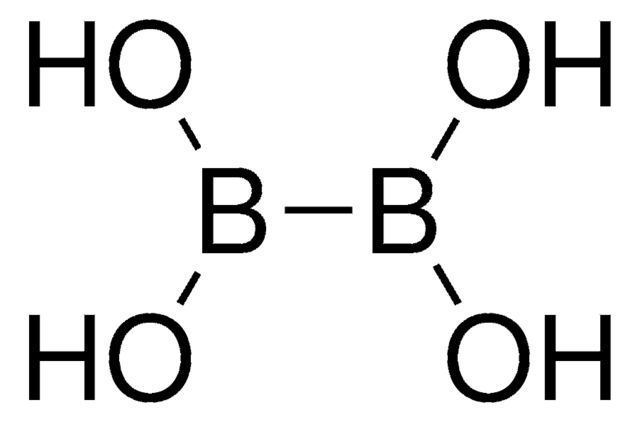
SMILES 字串
Nc1ccccc1-c2ccccc2[Pd]Cl.Nc3ccccc3-c4ccccc4[Pd]Cl.CC(C)c5cc(C(C)C)c(-c6cccc(c6)P(C7CCCCC7)C8CCCCC8)c(c5)C(C)C.CC(C)c9cc(C(C)C)c(c(c9)C(C)C)-c%10ccccc%10P(C%11CCCCC%11)C%12CCCCC%12
InChI
1S/2C33H49P.2C12H10N.2ClH.2Pd/c1-23(2)27-21-31(24(3)4)33(32(22-27)25(5)6)26-14-13-19-30(20-26)34(28-15-9-7-10-16-28)29-17-11-8-12-18-29;1-23(2)26-21-30(24(3)4)33(31(22-26)25(5)6)29-19-13-14-20-32(29)34(27-15-9-7-10-16-27)28-17-11-8-12-18-28;2*13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;;;;/h13-14,19-25,28-29H,7-12,15-18H2,1-6H3;13-14,19-25,27-28H,7-12,15-18H2,1-6H3;2*1-6,8-9H,13H2;2*1H;;/q;;;;;;2*+1/p-2
InChI 密鑰
HMRJFNBZAWHTGN-UHFFFAOYSA-L
相關類別
一般說明
應用
- 钯催化的有机三氟硼酸钾与氨基磺酸盐的Suzuki-Miyaura偶联反应。
- 敏感芳基和杂芳基硼酸的Suzuki-Miyaura交叉偶联反应。
- 通过Suzuki-Miyaura交叉偶联反应,合成钾Boc保护的仲氨基甲基三氟硼酸盐。
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
文章
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務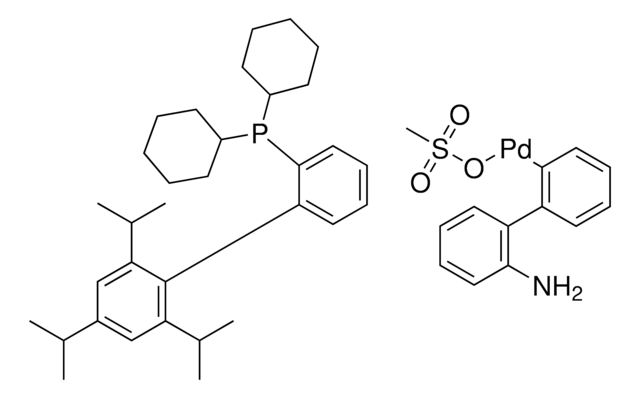

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)


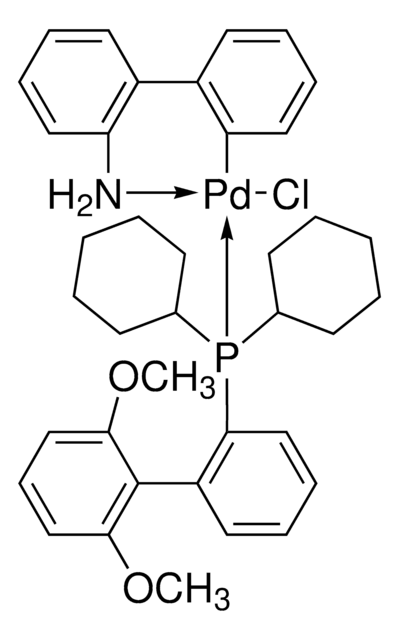
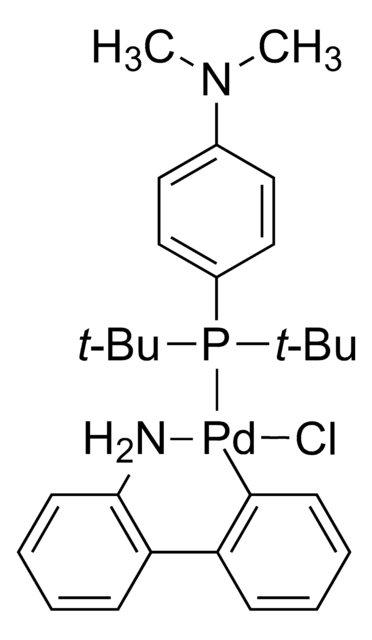
![氯[(三-TERT-三丁基膦)-2-(2-氨基联苯)]钯(II)](/deepweb/assets/sigmaaldrich/product/structures/100/957/42c5dad6-6197-4fa6-8481-3fe55f0291e9/640/42c5dad6-6197-4fa6-8481-3fe55f0291e9.png)
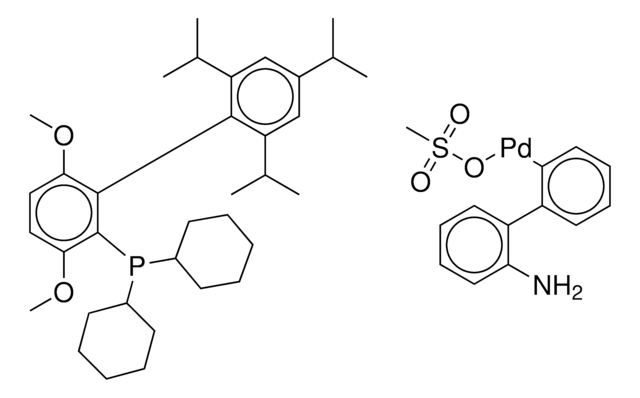
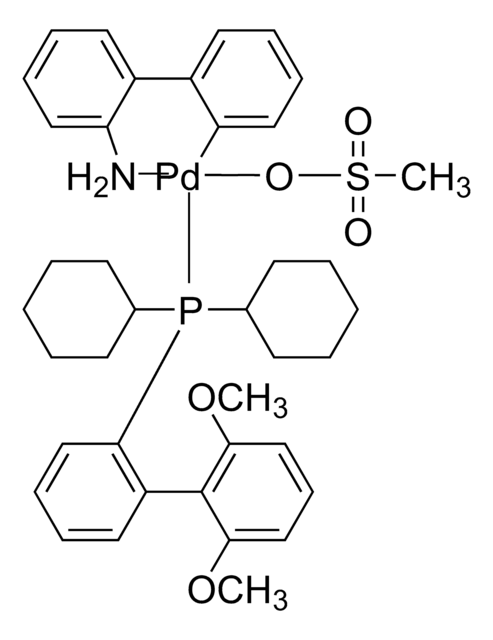
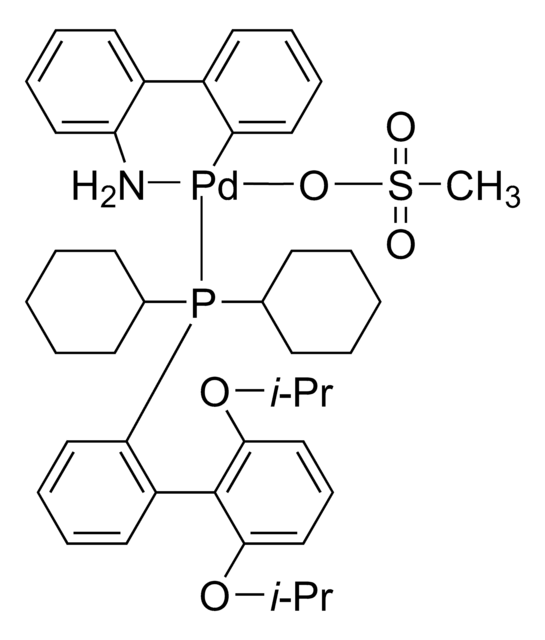
![氯(2-二环己基膦基-2′,6′-二甲氧基-1,1′-联苯基)[2-(2-氨基乙基苯基)]钯(II) - 甲基--叔丁基醚加合物](/deepweb/assets/sigmaaldrich/product/structures/421/182/4ca66fb3-8d93-499c-b88b-b8fe48ca97b8/640/4ca66fb3-8d93-499c-b88b-b8fe48ca97b8.png)