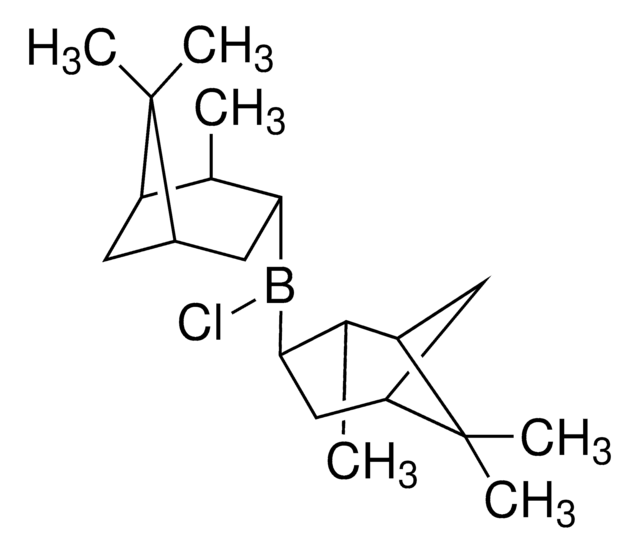
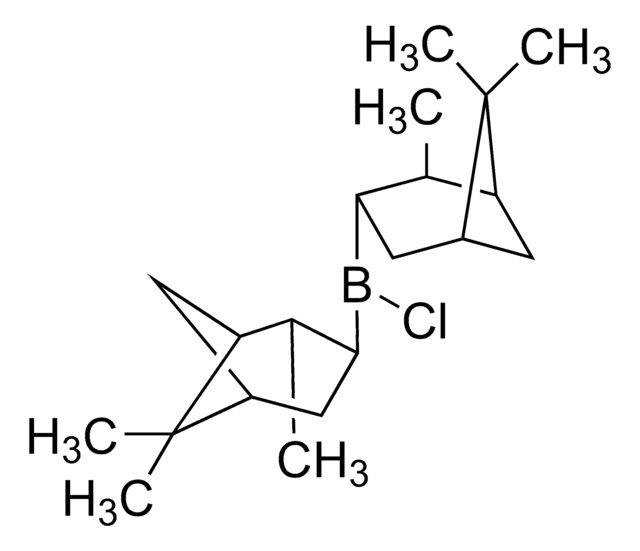
About This Item
推薦產品
光學活性
[α]20/D −35 to ±5°, c = 1
濃度
1 M in pentane
bp
35-36 °C
密度
0.638 g/mL at 25 °C
儲存溫度
−20°C
SMILES 字串
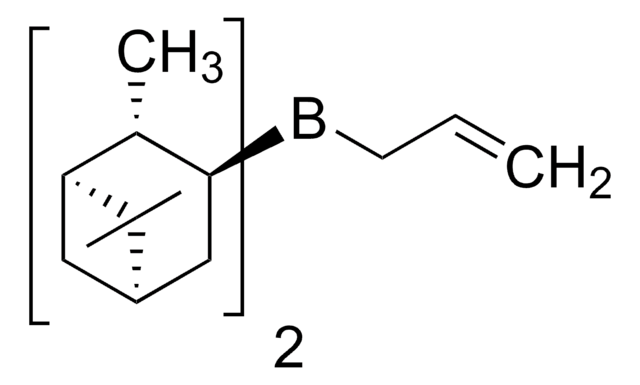
C[C@H]1[C@@H](C[C@@H]2C[C@H]1C2(C)C)B(CC=C)[C@@H]3C[C@@H]4C[C@H]([C@H]3C)C4(C)C
InChI
1S/C23H39B/c1-8-9-24(20-12-16-10-18(14(20)2)22(16,4)5)21-13-17-11-19(15(21)3)23(17,6)7/h8,14-21H,1,9-13H2,2-7H3/t14-,15-,16+,17+,18-,19-,20-,21-/m1/s1
InChI 密鑰
ZIXZBDJFGUIKJS-RLEROFIGSA-N
尋找類似的產品? 前往 產品比較指南
應用
其他說明
訊號詞
Danger
危險分類
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Central nervous system, Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
-56.2 °F - closed cup
閃點(°C)
-49 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves
客戶也查看了
文章
Asymmetric allylboration of aldehydes is an extremely important method for preparation of homoallylic alcohols, as evidenced in numerous complex natural product syntheses.
Asymmetric Allylboration Using Ipc2B(allyl)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務