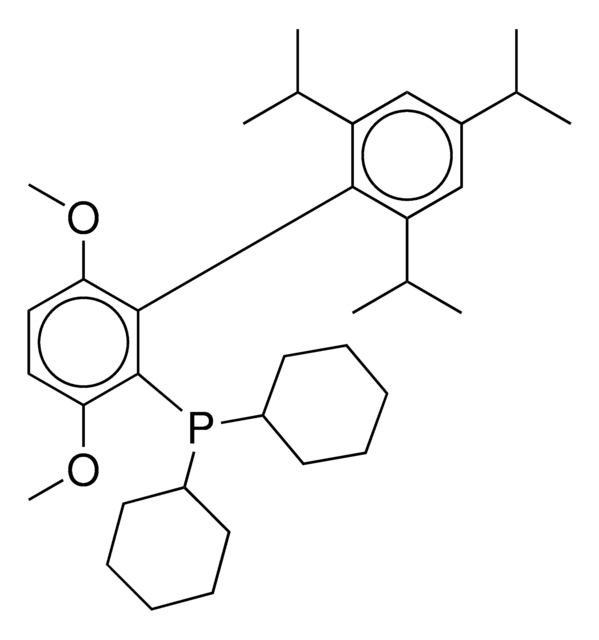
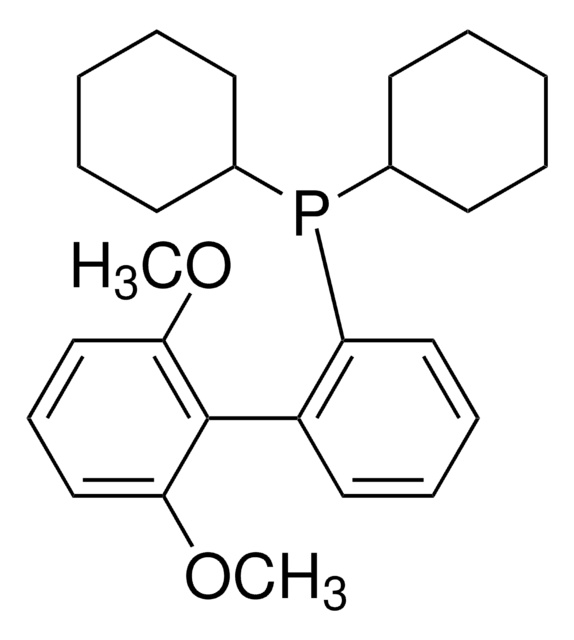
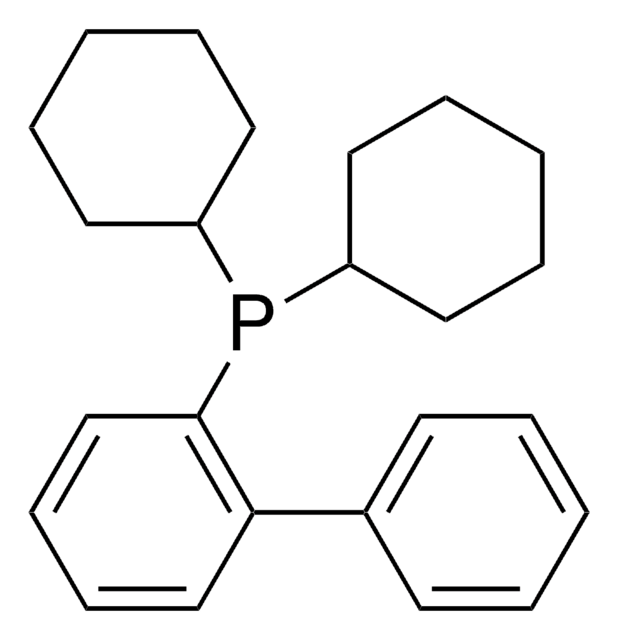
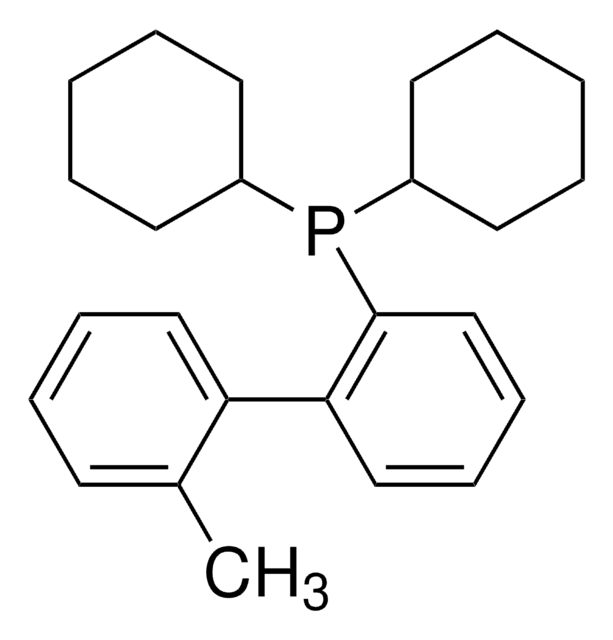
About This Item
推薦產品
品質等級
化驗
98%
形狀
solid
反應適用性
reaction type: Cross Couplings
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
環保替代產品評分
old score: 3
new score: 1
Find out more about DOZN™ Scoring
環保替代產品特色
Atom Economy
Design for Energy Efficiency
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
雜質
1-5% Bis(2′,6′-Diisopropoxybiphenyl)cyclohexylphosphine
mp
123-126 °C
官能基
phosphine
環保替代類別
SMILES 字串
CC(C)Oc1cccc(OC(C)C)c1-c2ccccc2P(C3CCCCC3)C4CCCCC4
InChI
1S/C30H43O2P/c1-22(2)31-27-19-13-20-28(32-23(3)4)30(27)26-18-11-12-21-29(26)33(24-14-7-5-8-15-24)25-16-9-6-10-17-25/h11-13,18-25H,5-10,14-17H2,1-4H3
InChI 密鑰
MXFYYFVVIIWKFE-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
法律資訊
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
文章
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 663131-25G | 4061833399507 |
| 663131-100G | 4061833301555 |
| 663131-1G | 4061832734279 |
| 663131-5G | 4061832734286 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務