495492
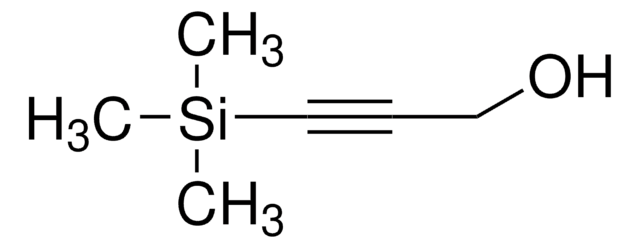
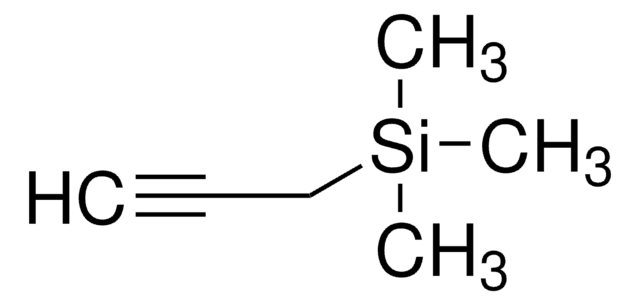
叔丁基二甲基(2-丙炔氧基)硅烷
97%
同義詞:
(1,1-Dimethylethyl)dimethyl(2-propyn-1-yloxy)silane, 1-(tert-Butyldimethylsilyloxy)-2-propyne, 3-(Dimethyl-tert-butylsiloxy)propyne, 3-(tert-Butyldimethylsilyloxy)-1-propyne, 3-(tert-Butyldimethylsilyloxy)propyne, 3-tert-Butyldimethylsiloxy-1-propyne, Dimethyl(2-Propynyloxy)(tert-butyl)silane
About This Item
推薦產品
品質等級
化驗
97%
折射率
n20/D 1.429 (lit.)
bp
40 °C/8 mmHg (lit.)
密度
0.84 g/mL at 25 °C (lit.)
SMILES 字串
CC(C)(C)[Si](C)(C)OCC#C
InChI
1S/C9H18OSi/c1-7-8-10-11(5,6)9(2,3)4/h1H,8H2,2-6H3
InChI 密鑰
ZYDKYFIXEYSNPO-UHFFFAOYSA-N
一般說明
應用
客戶也查看了
文章
Alkynes contain a highly versatile functional group that may be utilized for numerous reactions such as electrophilic additions of hydrogen, halogens, hydrogen halides, or water; metathesis; hydroboration; oxidative cleavage; C–C coupling; and cycloadditions
Alkynes contain a highly versatile functional group that may be utilized for numerous reactions such as electrophilic additions of hydrogen, halogens, hydrogen halides, or water; metathesis; hydroboration; oxidative cleavage; C–C coupling; and cycloadditions
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務