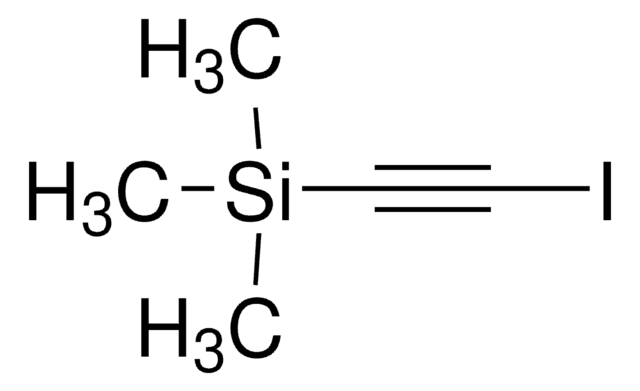494011
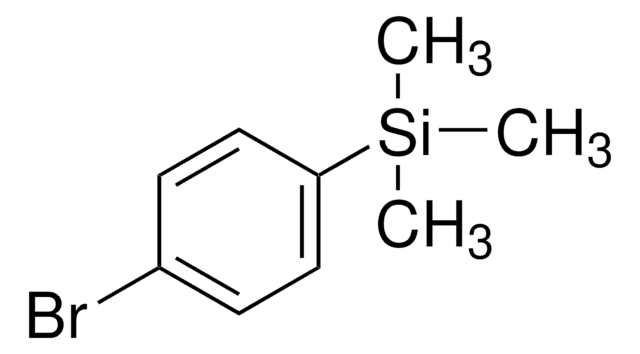
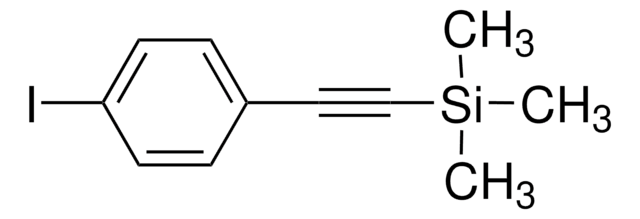
(4-溴苯乙炔基)三甲基硅烷
98%
同義詞:
(p-Bromophenyl)ethynyl)trimethylsilane, 1-(p-Bromophenyl)-2-trimethylsilylacetylene, 1-Bromo-4-(2-trimethylsilylethynyl)benzene, 1-Bromo-4-(trimethylsilylethynyl)benzene, 1-Bromo-4-[2-(trimethylsilyl)ethynyl]benzene, 2-(p-Bromophenyl)-1-trimethylsilylacetylene, 4-(Trimethylsilylethynyl)phenyl bromide, [(4-Bromophenyl)ethynyl]trimethylsilane, [1-(4-Bromophenyl)-2-trimethylsilyl]acetylene, [2-(4-Bromophenyl)ethynyl]trimethylsilane
登入查看組織和合約定價
全部照片(1)
About This Item
線性公式:
BrC6H4C≡CSi(CH3)3
CAS號碼:
分子量::
253.21
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
一般說明
(4-Bromophenylethynyl)trimethylsilane can be synthesized by the palladium catalyzed reaction between 4-bromo-1-iodobenzene and trimethylsilylacetylene. It undergoes Buchwald-Hartwig coupling with para-substituted diphenylamines.
應用
(4-Bromophenylethynyl)trimethylsilane may be used to synthesize:
- 1-bromo-4-ethynylbenzene
- 4-(4-bromophenyl)-3-butyn-2-one
- 4-ethynyl-4′-tert-butylbiphenyl
- 1,4-bis[2-(4-bromophenyl)ethynyl]-2,5-dihexylbenzene
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
Probing surface-adlayer conjugation on organic-modified Si (111) surfaces with microscopy, scattering, spectroscopy, and density functional theory.
Kellar JA, et al.
The Journal of Physical Chemistry C, 113(7), 2919-2927 (2009)
Experimental and computational probes of the space in a self-assembled capsule.
Ajami D, et al.
Proceedings of the National Academy of Sciences of the USA, 103(24), 8934-8936 (2006)
Synthesis and Solid-State Investigations of Oligo-Phenylene-Ethynylene Structures with Halide End-Groups.
Jenny NM, et al.
European Journal of Organic Chemistry, 2012(14), 2738-2747 (2012)
Tunable, strongly-donating perylene photosensitizers for dye-sensitized solar cells.
Mathew S and Imahori H.
Journal of Materials Chemistry, 21(20), 7166-7174 (2011)
Enantioselective synthesis of both enantiomers of various propargylic alcohols by use of two oxidoreductases.
Schubert T, et al.
European Journal of Organic Chemistry, 2001(22), 4181-4187 (2001)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務