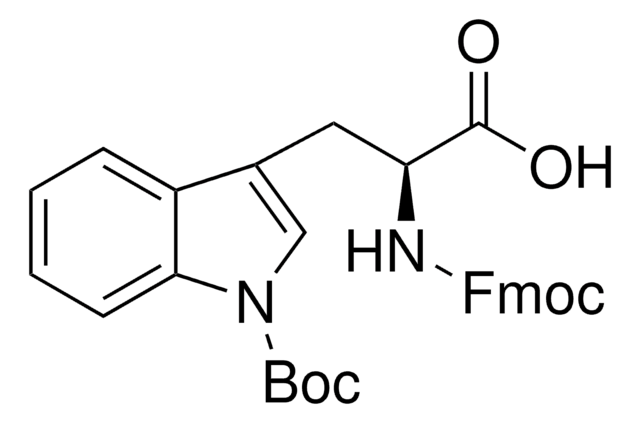
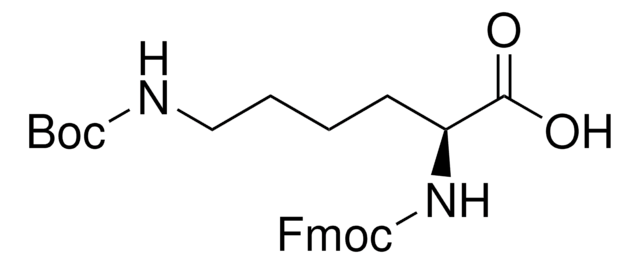
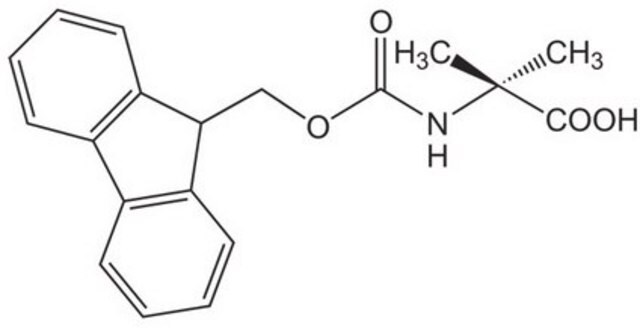
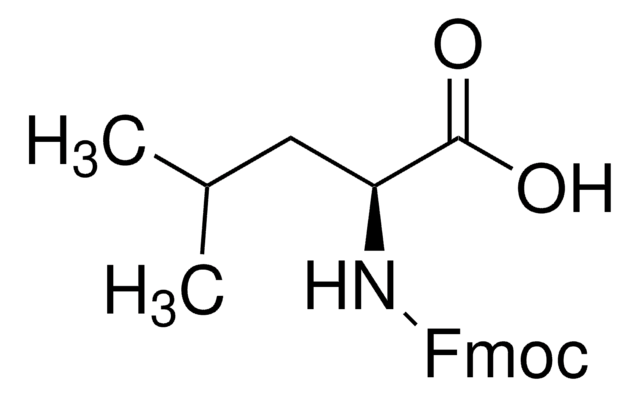
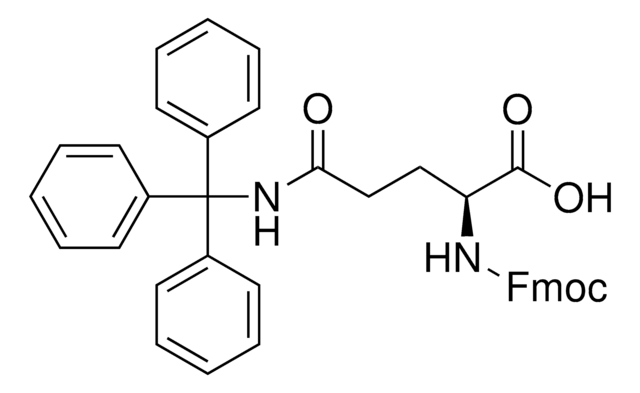
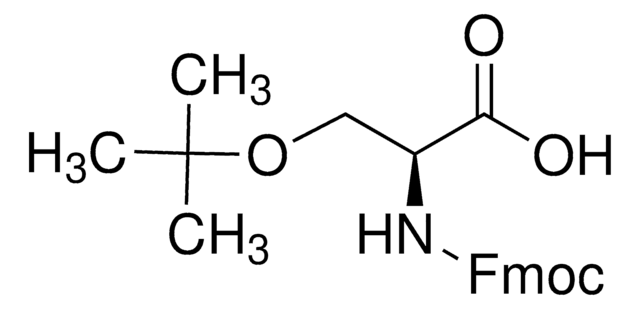
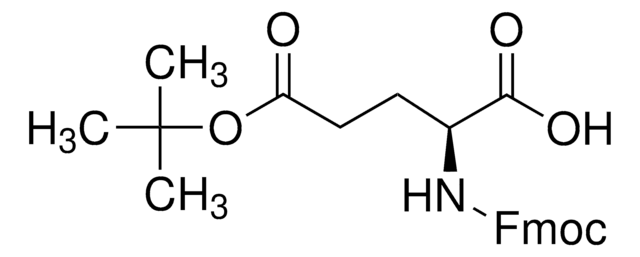
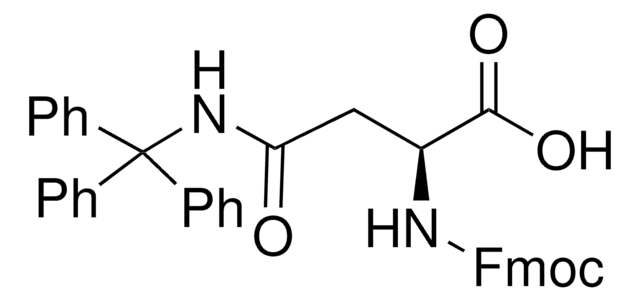
推薦產品
產品名稱
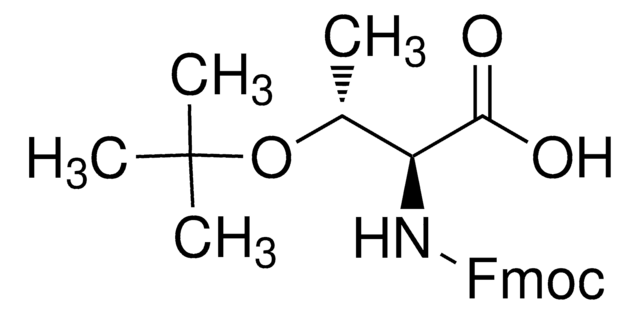
Fmoc-Tyr(tBu)-OH, ≥98.0% (HPLC)
化驗
≥98.0% (HPLC)
形狀
powder
光學活性
[α]20/D −29±2°, c = 1% in DMF
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
mp
153-156 °C (lit.)
應用
peptide synthesis
官能基
Fmoc
儲存溫度
2-8°C
SMILES 字串
CC(C)(C)Oc1ccc(C[C@H](NC(=O)OCC2c3ccccc3-c4ccccc24)C(O)=O)cc1
InChI
1S/C28H29NO5/c1-28(2,3)34-19-14-12-18(13-15-19)16-25(26(30)31)29-27(32)33-17-24-22-10-6-4-8-20(22)21-9-5-7-11-23(21)24/h4-15,24-25H,16-17H2,1-3H3,(H,29,32)(H,30,31)/t25-/m0/s1
InChI 密鑰
JAUKCFULLJFBFN-VWLOTQADSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
文章
With a growing peptide drug market the fast, reliable and uncomplicated synthesis of peptides is of paramount importance.
With a growing peptide drug market the fast, reliable and uncomplicated synthesis of peptides is of paramount importance.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務