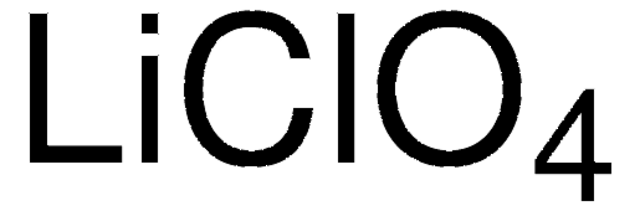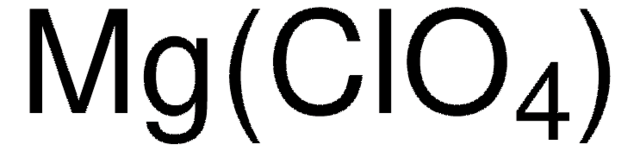推薦產品
等級
ACS reagent
品質等級
化驗
99.99% trace metals basis
形狀
granular
反應適用性
reagent type: oxidant
環保替代產品特色
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
雜質
≤0.005% insolubles
<100 ppm total metallic impurities
pH值
6.0-7.5
mp
236 °C (lit.)
溶解度
H2O: 106.4 g/L at 20 °C
負離子痕跡
chloride (Cl-): ≤0.003%
sulfate (SO42-): ≤0.001%
環保替代類別
SMILES 字串
[Li+].[O-]Cl(=O)(=O)=O
InChI
1S/ClHO4.Li/c2-1(3,4)5;/h(H,2,3,4,5);/q;+1/p-1
InChI 密鑰
MHCFAGZWMAWTNR-UHFFFAOYSA-M
尋找類似的產品? 前往 產品比較指南
一般說明
我们竭诚为您带来符合一项或多项绿色化学12项原则要求的绿色替代产品。该产品为增强型,提高了能源效率。点击此处以获取更多信息。
高氯酸锂是一种白色的盐,很容易溶于水。由于其优异的电阻抗、导电性、吸湿性和阳极稳定性,它通常被用作电池中的电解质盐。这些特性对其在电池应用中的作用至关重要。此外,它是一种强氧化剂,因此在固体火箭推进剂和有机反应中用作氧化剂。
應用
高氯酸锂可用于:
- 作为制备用于可充电锂离子电池的固体聚合物电解质的前体。
- 作为制备聚合物基固体推进剂的氧化剂。
- 制备用于染料敏化太阳能电池(DSSC)的硫化钴(CoS)基对电极。
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1A - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
5.1A - Strongly oxidizing hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
Shokaku Kim et al.
Organic letters, 4(21), 3735-3737 (2002-10-12)
[reaction: see text] N-Acyliminium cation of prolines was efficiently generated to accumulate in an undivided cell at 0 degrees C by an anodic oxidation of N-acylprolines or alpha'-phenylsulfanylated N-acylproline derivatives in a lithium perchlorate/nitromethane solution. The iminium cation intermediates gave
L H Sim et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 76(3-4), 287-292 (2010-05-07)
The interaction behaviours between components of polyacrylate (PAc)/poly(ethylene oxide) (PEO) and lithium perchlorate (LiClO(4)) were investigated in detail by Attenuated Total Reflectance (ATR)-Fourier Transformed Infrared (FTIR) spectroscopy. Solution cast films of the PAc/PEO and PAc/PEO/LiClO(4) were examined. No obvious shifting
Natalia Varaksa et al.
Proceedings of the National Academy of Sciences of the United States of America, 99(8), 5012-5017 (2002-04-18)
The adsorption of the trigonal connector, 1,3,5-tris[10-(3-ethylthiopropyl)dimethylsilyl-1,10-dicarba-closo-decaboran-1-yl]benzene (1), from acetonitrile/0.1 M LiClO(4) on the surface of mercury at potentials ranging from +0.3 to -1.4 V (vs. aqueous Ag/AgCl/1 M LiCl) was examined by voltammetry, Langmuir isotherms at controlled potentials, and
Witold Darlewski et al.
Journal of hazardous materials, 175(1-3), 460-467 (2009-11-17)
A process of dibutyl sulphide (DBS) electro-oxidation using electrolysis and cyclic voltamperometry was investigated in water-methanol solution using different electrodes (platinum, boron doped diamond, graphite and glassy carbon). Obtained results indicate that the DBS electro-oxidation process is irreversible in voltamperometric
Francisco Palacios et al.
The Journal of organic chemistry, 67(7), 2131-2135 (2002-04-02)
Functionalized keto-enamines 6 were obtained by nucleophilic addition of enol ethers to the imine moiety of 2-azadienes derived from dehydroaspartic esters 4. Reactions of 2-azadiene 4c containing three electron-withdrawing substituents (CO(2)R) with enol ethers 5 in the presence of lithium
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務