全部照片(1)
About This Item
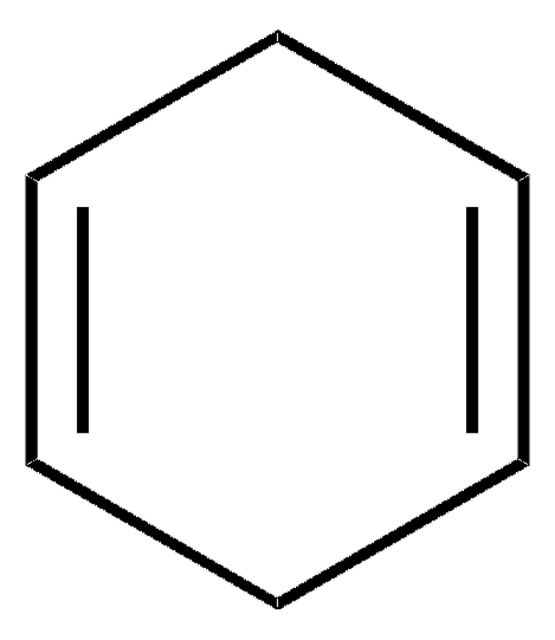
經驗公式(希爾表示法):
C9H16OSi
CAS號碼:
分子量::
168.31
Beilstein:
2079140
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
化驗
95%
形狀
liquid
折射率
n20/D 1.462 (lit.)
bp
65 °C/7 mmHg (lit.)
密度
0.899 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
C[Si](C)(C)OC1=CCCC=C1
InChI
1S/C9H16OSi/c1-11(2,3)10-9-7-5-4-6-8-9/h5,7-8H,4,6H2,1-3H3
InChI 密鑰
WPIRVUXAMPRMAY-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
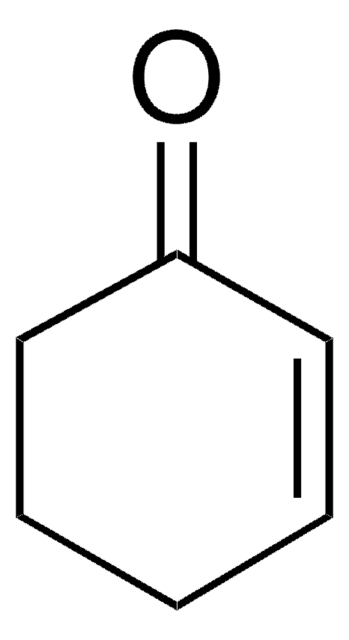
2-(Trimethylsiloxy)-1,3-cyclohexadiene ((cyclohexa-1,5-dien-1-yloxy)trimethylsilane) is silyl enol ether of cyclohexenone. The Diels–Alder reactions with dienophiles α-acetoxyacrylonitrile, acrylonitrile and α-chloroacrylonitrile has been studied.
應用
2-(Trimethylsiloxy)-1,3-cyclohexadiene may be used as a diene in the synthesis of isoquinuclidinone 2-aza[2.2.2]octa-3,5-dione by reacting with p-toluenesulfonyl cyanide.
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Safety Assessment of Diels-Alder Reactions with Highly Reactive Acrylic Monomers.
Abele S, et al.
Organic Process Research & Development, 16(12), 2015-2020 (2012)
Design and Scale-Up of Diels-Alder Reactions for the Practical Synthesis of 5-Phenylbicyclo [2.2. 2] oct-5-en-2-one.
Funel JA, et al.
Organic Process Research & Development, 15(6), 1420-1427 (2011)
High-Temperature Diels-Alder Reactions: Transfer from Batch to Continuous Mode.
Abele S, et al.
Organic Process Research & Development, 16(5), 1114-1120 (2011)
Cynthia K McClure et al.
The Journal of organic chemistry, 68(21), 8256-8257 (2003-10-11)
The hetero-Diels-Alder reaction of an electron-deficient nitrile, p-toluenesulfonyl cyanide, with the silyl enol ether of cyclohexenone produced a hydrolytically sensitive [4 + 2] adduct in good yield. Use of Mander's reagent, ethyl cyanoformate, with the same diene, produced an unstable
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務