全部照片(2)
About This Item
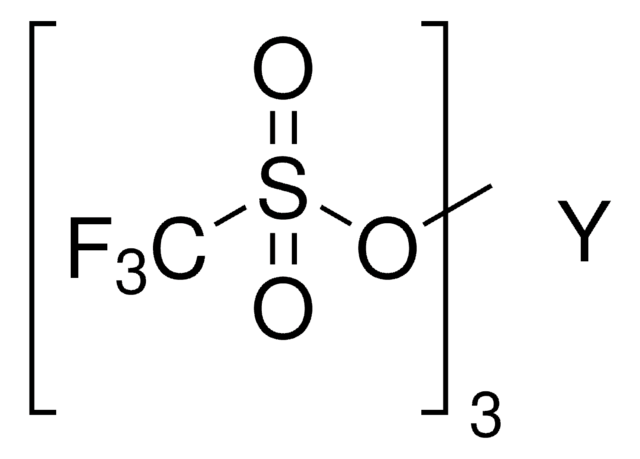
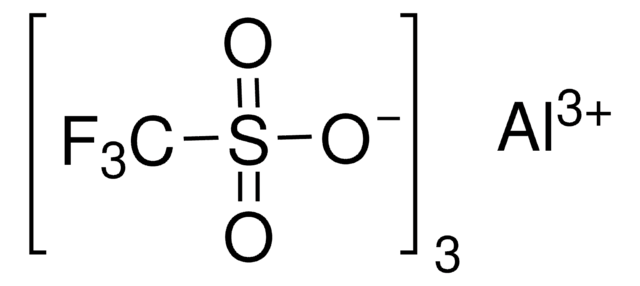
線性公式:
Sc(SO3CF3)3
CAS號碼:
分子量::
492.16
Beilstein:
8510151
MDL號碼:
分類程式碼代碼:
12161600
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
99%
形狀
powder
反應適用性
core: scandium
reagent type: catalyst
SMILES 字串
[Sc+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Sc/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
InChI 密鑰
HZXJVDYQRYYYOR-UHFFFAOYSA-K
尋找類似的產品? 前往 產品比較指南
一般說明
三氟甲磺酸钪是一种非常活跃、高效、可回收和可重复使用的酰化催化剂。三氟甲磺酸钪是弗里德尔-克拉夫茨酰化反应、狄尔斯-阿尔德反应和其他碳-碳键形成反应的重要催化剂。 它还可以立体化学催化丙烯酸酯的自由基聚合反应。(4′S,5′S)-2,6-双[4′-(三异丙基)氧甲基-5′-苯基-1′,3′-恶唑啉-2′-yl]吡啶的三氟甲磺酸钪络合物,已被用作取代吲哚与甲基(E)-2-氧代-4-芳基-3-丁烯酸甲酯之间的不对称弗里德尔-克拉夫茨反应的催化剂。
應用
三氟甲磺酸钪作为催化剂用于:
- 芳香族和脂肪族硫醇的加氢硫醇反应。
- 二茂铁衍生物对O2的选择性双电子还原。
- 吲哚和吡咯在水中的烷基化反应。
- β腈酮的合成。
- 与三乙基硅烷结合,通过还原方式开放功能化的吡喃糖苷环。
- 通过稳定的硫叶立德合成牛酮的关键步骤。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Kazuaki Ishihara et al.
The Journal of organic chemistry, 61(14), 4560-4567 (1996-07-12)
Scandium trifluoromethanesulfonate (triflate), which is commercially available, is a practical and useful Lewis acid catalyst for acylation of alcohols with acid anhydrides or the esterification of alcohols by carboxylic acids in the presence of p-nitrobenzoic anhydrides. The remarkably high catalytic
Krzysztof Kuciński et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(13), 4940-4943 (2015-02-18)
The first use of a Lewis acid catalyst in the addition reaction of both aromatic and aliphatic thiols to unsaturated organosilicon compounds is reported. In catalytic tests, scandium(III) triflate demonstrates high catalytic activity in this process. Under mild conditions (25 °C
Saya Kakuda et al.
Journal of the American Chemical Society, 137(9), 3330-3337 (2015-02-11)
Mononuclear copper complexes, [(tmpa)Cu(II)(CH3CN)](ClO4)2 (1, tmpa = tris(2-pyridylmethyl)amine) and [(BzQ)Cu(II)(H2O)2](ClO4)2 (2, BzQ = bis(2-quinolinylmethyl)benzylamine)], act as efficient catalysts for the selective two-electron reduction of O2 by ferrocene derivatives in the presence of scandium triflate (Sc(OTf)3) in acetone, whereas 1 catalyzes
Okamoto, Y., et al.
Macromolecular Symposia, 183, 83-83 (2002)
Jens Oelerich et al.
Organic & biomolecular chemistry, 13(9), 2793-2799 (2015-01-22)
Alkylidene malonates and α,β-unsaturated α'-hydroxyketones are demonstrated to be efficient classes of electrophiles for the scandium(III) triflate/sodium dodecyl sulphate (SDS) catalysed vinylogous Friedel-Crafts alkylation of indoles and pyrroles in water. These substrates contain an easily removable auxiliary group that increases
文章
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務