全部照片(2)
About This Item
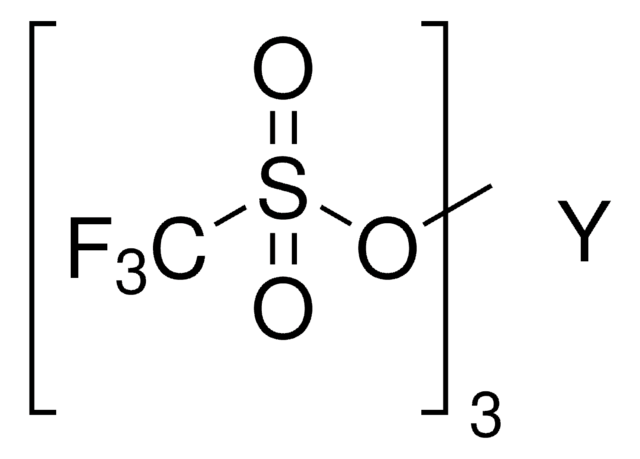
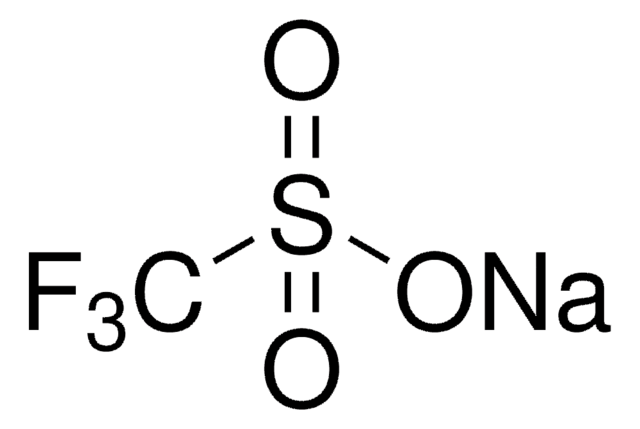
線性公式:
(CF3SO3)3Yb · xH2O
CAS號碼:
分子量::
620.25 (anhydrous basis)
MDL號碼:
分類程式碼代碼:
12161600
PubChem物質ID:
NACRES:
NA.22
推薦產品
成份
Degree of hydration, 1-2
Yb, 25-28% (approx.)
反應適用性
core: ytterbium
reagent type: catalyst
SMILES 字串
[H]O[H].FC(F)(F)S(=O)(=O)O[Yb](OS(=O)(=O)C(F)(F)F)OS(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.H2O.Yb/c3*2-1(3,4)8(5,6)7;;/h3*(H,5,6,7);1H2;/q;;;;+3/p-3
InChI 密鑰
BUJKNFNMGRYZBV-UHFFFAOYSA-K
尋找類似的產品? 前往 產品比較指南
一般說明
已经通过原子力显微镜(AFM)研究了4-氨基苯硫酚和三氟甲磺酸镱(III)水合物膜的逐层组装。 据报道,双(恶唑啉基)-吡啶- 钪(III) 三氟甲磺酸盐络合物能够催化各种吲哚的对映选择性Friedel-Crafts加成反应。
應用
三氟甲磺酸镱(III)水合物(Yb(OTf)3)已被用作Lewis酸催化剂,用于以下研究:
- 通过Biginelli环缩合反应,合成二氢嘧啶(DHPM)衍生物monastrol。
- 酮与醛的交叉醛醇反应。
- 合成β-酮烯醇醚。
- 在新型立体选择性分子内Diels-Alder反应中,合成deoxypenostatin A。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Ytterbium Trifluoromethanesulfonate Mediated Cross-Aldol Reaction between Ketones and Aldehydes.
Fukuzawa S-I, et al.
Bulletin of the Chemical Society of Japan, 67(8), 2227-2232 (1994)
David A Evans et al.
Journal of the American Chemical Society, 129(32), 10029-10041 (2007-07-31)
The enantioselective Friedel-Crafts addition of a variety of indoles catalyzed by bis(oxazolinyl)pyridine-scandium(III) triflate complexes (Sc(III)-pybox) was accomplished utilizing a series of beta-substituted alpha,beta-unsaturated phosphonates and alpha,beta-unsaturated 2-acyl imidazoles. The acyl phosphonate products were efficiently transformed into esters and amides, whereas
Ytterbium triflate catalyzed synthesis of β-keto enol ethers.
Curini M, et al.
Tetrahedron Letters, 47(27), 4697-4700 (2006)
Doris Dallinger et al.
Nature protocols, 2(2), 317-321 (2007-04-05)
We present here a protocol for the synthesis of the dihydropyrimidine (DHPM) derivative monastrol, which is known to be a specific mitotic kinesin Eg5 inhibitor. By applying controlled microwave heating under sealed-vessel conditions, the synthesis via the one-pot three-component Biginelli
Snider, B.B. Liu, T.
The Journal of Organic Chemistry, 64, 1088-1088 (1999)
文章
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務