全部照片(3)
About This Item
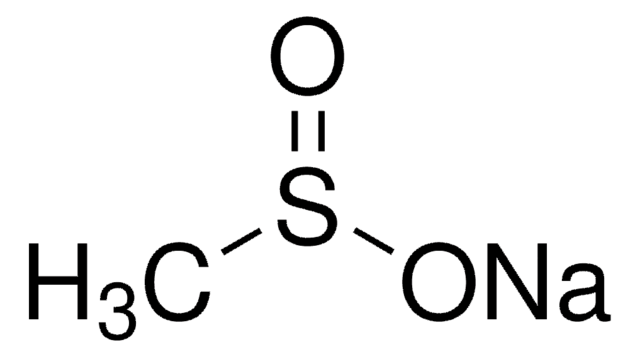
線性公式:
(CF3SO3Cu)2 · C6H6
CAS號碼:
分子量::
503.34
EC號碼:
MDL號碼:
分類程式碼代碼:
12161600
PubChem物質ID:
NACRES:
NA.22
推薦產品
等級
technical grade
品質等級
化驗
90%
形狀
powder
反應適用性
core: copper
reagent type: catalyst
mp
160 °C (dec.) (lit.)
SMILES 字串
[Cu+].[Cu+].c1ccccc1.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/C6H6.2CHF3O3S.2Cu/c1-2-4-6-5-3-1;2*2-1(3,4)8(5,6)7;;/h1-6H;2*(H,5,6,7);;/q;;;2*+1/p-2
InChI 密鑰
GNXZWVVAAMVOJY-UHFFFAOYSA-L
應用
三氟甲磺酸铜(I)苯络合物可用作以下反应的催化剂:
它还可与基于氨基酸的手性膦配体结合使用,催化烷基锌与无环α,β-不饱和酮的不对称共轭加成,以高产率和优异的对映选择性合成β-烷基羰基。
- 通过形成羧酸铜(I)中间体,合成烯醇酯。
- 环状烯烃的对映选择性烯丙基氧化。
- 通过形成磺酰胺基自由基,由N-烯基、炔基和烷基N-苯甲酰氧基磺酰胺制备2,5-二取代的吡咯烷衍生物。
它还可与基于氨基酸的手性膦配体结合使用,催化烷基锌与无环α,β-不饱和酮的不对称共轭加成,以高产率和优异的对映选择性合成β-烷基羰基。
訊號詞
Warning
危險聲明
危險分類
Flam. Sol. 2
儲存類別代碼
4.1B - Flammable solid hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Jerome Bayardon et al.
The Journal of organic chemistry, 69(9), 3121-3128 (2004-04-24)
Various enantiopure fluorous bis(oxazolines) with fluorine content between 52.7 and 58.7% have been synthesized by a simple reaction sequence that involved the introduction of two fluorinated ponytails by alkylation of the corresponding nonfluorous bis(oxazolines). These new ligands have been used
Enantioselective total synthesis of erogorgiaene: applications of asymmetric Cu-catalyzed conjugate additions of alkylzincs to acyclic enones
Cesati RR, et al.
Journal of the American Chemical Society, 126(1), 96-101 (2004)
Synthesis of Enol Esters from Copper (I) Carboxylates Generated from Copper (I) Trifluoromethanesulfonate Benzene Complex
Lefler SR and Rose SD
Synthetic Communications, 29(21), 3805-3810 (1999)
Richard R Cesati et al.
Journal of the American Chemical Society, 126(1), 96-101 (2004-01-08)
The first enantioselective synthesis of erogorgiaene (1), an inhibitor of mycobacterium tuberculosis, is disclosed. The total synthesis highlights the utility of asymmetric conjugate additions (ACA) of alkylzincs to acyclic alpha,beta-unsaturated ketones catalyzed by peptidic phosphine ligands and (CuOTf)(2).C(6)H(6). Moreover, several
Enantiopure fluorous bis (oxazolines): Synthesis and applications in catalytic asymmetric reactions
Bayardon J and Sinou D
The Journal of Organic Chemistry, 69(9), 3121-3128 (2004)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務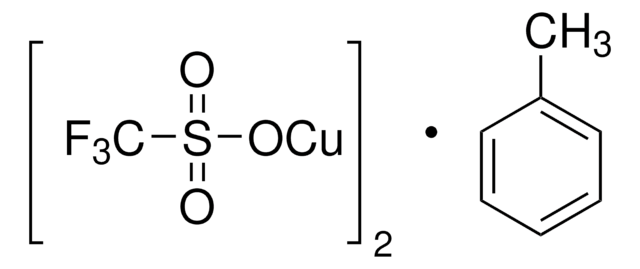

![氯[1,3-双(2,6-二异丙基苯基)咪唑-2-亚基]铜(I)](/deepweb/assets/sigmaaldrich/product/structures/199/763/44637b2e-b87c-42a3-abc3-3985b6cd7d5d/640/44637b2e-b87c-42a3-abc3-3985b6cd7d5d.png)