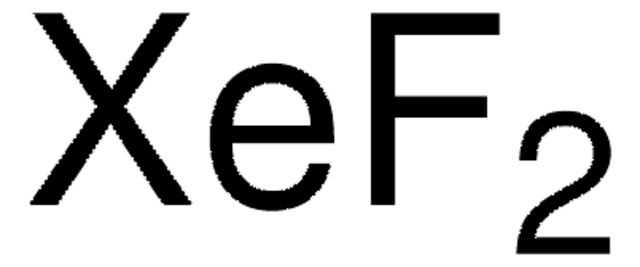推薦產品
蒸汽壓力
3.8 mmHg ( 25 °C)
化驗
99.99% trace metals basis
形狀
crystals
mp
129 °C (lit.)
密度
4.32 g/mL at 25 °C (lit.)
SMILES 字串
F[Xe]F
InChI
1S/F2Xe/c1-3-2
InChI 密鑰
IGELFKKMDLGCJO-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
在 473-523 oC 和 5 个绝对大气压下,氟化氙可以通过元素氙与氟相互作用得到。二氟化氙容易与路易斯酸相互作用,形成络合物。
應用
非常有用的氟化剂。氟化氙可用作氟化剂,通过气相色谱分析硫、硒和碲。
包裝
PFA/FEP 瓶封装
訊號詞
Danger
危險分類
Acute Tox. 1 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1B
儲存類別代碼
5.1B - Oxidizing hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
Use of Xenon difluoride for the determination of sulfur, selenium and tellurium as the volatile fluorides by gas chromatography
Aleinikov NN, et al.
Russian Chemical Bulletin, 22(11), 2552-2554 (1973)
Infrared spectra of complex compounds of xenon difluoride with ruthenium pentafluoride
Prusakov VN, et al.
Journal of Applied Spectroscopy, 17(1), 920-922 (1972)
Fluorination with XeF(2).(1) 44. Effect of Geometry and Heteroatom on the Regioselectivity of Fluorine Introduction into an Aromatic Ring.
Marko Zupan et al.
The Journal of organic chemistry, 63(3), 878-880 (2001-10-24)
Tsung Yi Chiang et al.
Journal of synchrotron radiation, 17(1), 69-74 (2009-12-24)
The synchrotron radiation (SR) stimulated etching of silicon elastomer polydimethylsiloxane (PDMS) using XeF(2) as an etching gas has been demonstrated. An etching system with differential pumps and two parabolic focusing mirrors was constructed to perform the etching. The PDMS was
Minseob Kim et al.
Nature chemistry, 2(9), 784-788 (2010-08-24)
The application of pressure, internal or external, transforms molecular solids into extended solids with more itinerant electrons to soften repulsive interatomic interactions in a tight space. Examples include insulator-to-metal transitions in O(2), Xe and I(2), as well as molecular-to-non-molecular transitions
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務