推薦產品
形狀
solid
mp
198-202 °C (dec.) (lit.)
官能基
nitro
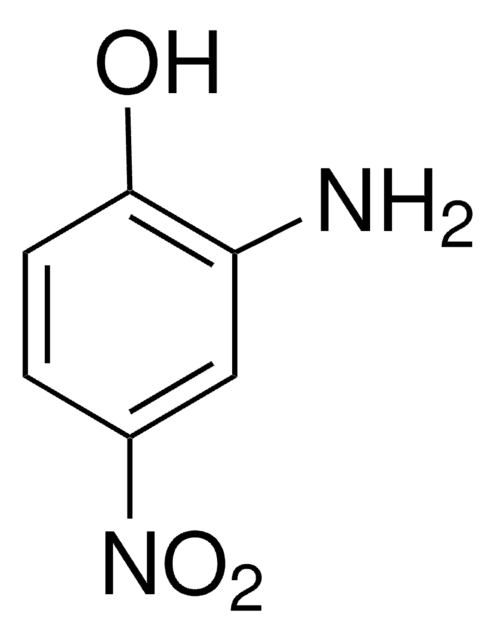
SMILES 字串
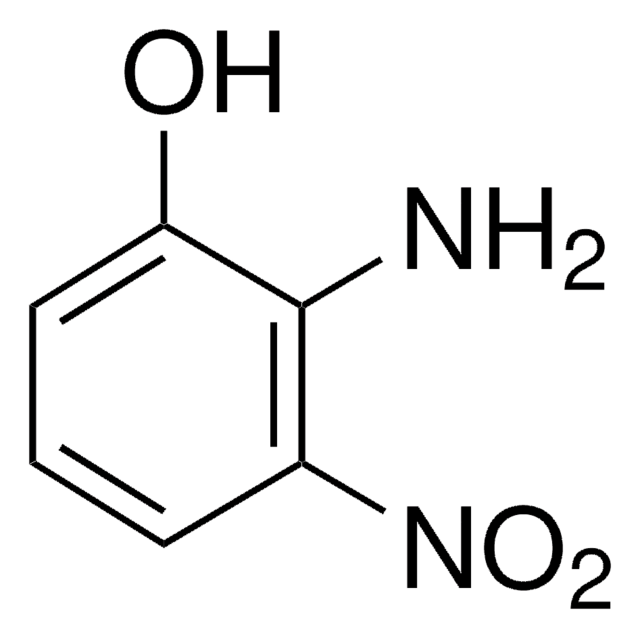
Nc1ccc(cc1O)[N+]([O-])=O
InChI
1S/C6H6N2O3/c7-5-2-1-4(8(10)11)3-6(5)9/h1-3,9H,7H2
InChI 密鑰
DOPJTDJKZNWLRB-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
2-氨基-5-硝基苯酚可用于:
- 作为合成(Z)-2-(取代芳基)-N-(3-氧代-4-(取代氨基甲硫基)-3,4-二氢-2H-苯并[b] [1,4]恶嗪-7-基)肼羧酰胺的起始原料
- 作为半永久性(非氧化)染发剂和永久性(氧化)染发产品中的色粉
- 合成7-苄氨基-2H-1,4-苯并恶嗪-3(4H)-酮系列化合物(抗惊厥药)
用于以下反应的反应物:
- 重氮化和偶联反应
- 生物学和药理活性分子的制备
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Nadeem Siddiqui et al.
Archiv der Pharmazie, 343(11-12), 657-663 (2010-11-27)
A series of (Z)-2-(substituted aryl)-N-(3-oxo-4-(substituted carbamothioyl)-3,4-dihydro-2H-benzo[b][1,4]oxazin-7-yl) hydrazine carboxamides (6a-r) was synthesized using 2-amino-5-nitrophenol as a starting material. All the synthesized compounds possessed two hydrogen-bonding domains and their effect on the activity was studied thereof. The anticonvulsant activity was assessed by
Christina L Burnett et al.
International journal of toxicology, 28(6 Suppl 2), 217S-251S (2010-01-30)
2-Amino-3-nitrophenol, 2-amino-4-nitrophenol, 2-amino-5-nitrophenol, 4-amino-3-nitrophenol, 4-amino-2-nitrophenol, 2-amino-4-nitrophenol sulfate, 3-nitro-p-hydroxyethylaminophenol, and 4-hydroxypropylamino-3-nitrophenol are substituted aromatic compounds used as semipermanent (nonoxidative) hair colorants and as toners in permanent (oxidative) hair dye products. All ingredients in this group except 2-amino-4-nitrophenol sulfate, 2-amino-5-nitrophenol, and 4-amino-2-nitrophenol
F Chen et al.
Cancer letters, 126(1), 67-74 (1998-05-01)
Two hair dye components, carcinogenic 4-nitro-2-aminophenol and 5-nitro-2-aminophenol, induced Cu(II)-dependent DNA cleavage frequently at thymine and guanine residues in DNA fragments obtained from the c-Ha-ras-1 protooncogene. When the p53 tumor suppressor gene was used, 4-nitro-2-aminophenol caused Cu(II)-dependent piperidine-labile sites at
2-Amino-5-nitrophenol.
IARC monographs on the evaluation of carcinogenic risks to humans, 57, 177-184 (1993-01-01)
Zhong-Tai Piao et al.
European journal of medicinal chemistry, 43(6), 1216-1221 (2007-10-24)
A series of 7-benzylamino-2H-1,4-benzoxazin-3(4H)-ones were synthesized using 2-amino-5-nitrophenol as a starting material. Their anticonvulsant activities were evaluated by the maximal electroshock test (MES test) and their neurotoxicity was evaluated by the rotarod neurotoxicity test (Tox.). The MES test showed that
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務