推薦產品
化驗
97%
折射率
n20/D 1.478 (lit.)
bp
104-106 °C/3.5 mmHg (lit.)
密度
1.085 g/mL at 25 °C (lit.)
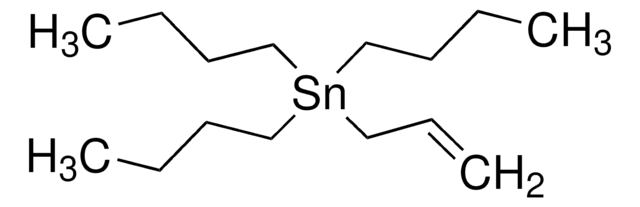
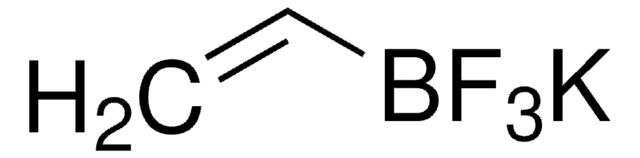
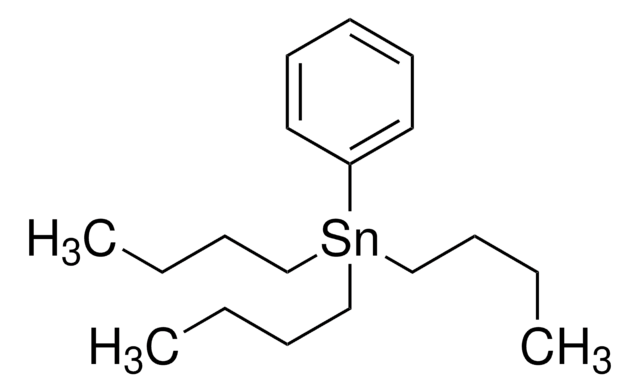
SMILES 字串
CCCC[Sn](CCCC)(CCCC)C=C
InChI
1S/3C4H9.C2H3.Sn/c3*1-3-4-2;1-2;/h3*1,3-4H2,2H3;1H,2H2;
InChI 密鑰
QIWRFOJWQSSRJZ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Flam. Liq. 3 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
109.4 °F - closed cup
閃點(°C)
43 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
文章
Reagents for C–C Bond Formation
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)