全部照片(1)
About This Item
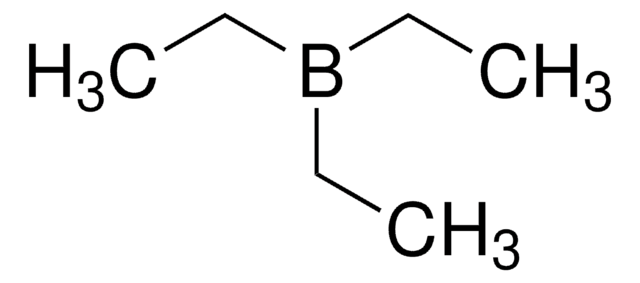
線性公式:
(C2H5)3B
CAS號碼:
分子量::
97.99
Beilstein:
1731462
MDL號碼:
分類程式碼代碼:
12352001
PubChem物質ID:
NACRES:
NA.22
推薦產品
形狀
liquid
反應適用性
reagent type: reductant
濃度
1.0 M in hexanes
密度
0.675 g/mL at 25 °C
SMILES 字串
CCB(CC)CC
InChI
1S/C6H15B/c1-4-7(5-2)6-3/h4-6H2,1-3H3
InChI 密鑰
LALRXNPLTWZJIJ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
三乙基硼烷可用于:
- 在水介质中作为自由基反应的自由基引发剂和终止剂。(1)
- 通过与2-取代的烯丙基胂叶立德反应合成聚(2-取代-1-亚丙烯基)聚合物。(2)
作为催化剂用于:
N -杂环卡宾硼烷还原烷基溴的反应物
具有氧化电位的四甲基铵三烷基苯硼酸酯盐合成用反应物
- 醛的烯丙基化
- 脱羧酶 C-C 键断裂反应
- 氢化铼/硼路易斯酸共催化烯烃加氢反应
- 不饱和肟醚的区域选择性羟基烷基化
N -杂环卡宾硼烷还原烷基溴的反应物
具有氧化电位的四甲基铵三烷基苯硼酸酯盐合成用反应物
訊號詞
Danger
危險分類
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1A - STOT RE 1 Inhalation - STOT SE 3
標靶器官
Central nervous system, Nervous system
儲存類別代碼
4.3 - Hazardous materials which set free flammable gases upon contact with water
水污染物質分類(WGK)
WGK 3
閃點(°F)
-32.8 °F
閃點(°C)
-36 °C
Synthesis of poly (2-substituted-1-propenylene)s from allylic arsonium ylides.
Mondiere R, et al.
Macromolecules, 38(3), 663-668 (2005)
Free-radical reaction of imine derivatives in water.
Miyabe H, et al.
The Journal of Organic Chemistry, 65(16), 5043-5047 (2000)
Hideto Miyabe et al.
Chemical & pharmaceutical bulletin, 51(5), 540-544 (2003-05-09)
Stereocontrol in radical reactions of oxime ether anchored to polymer support was studied. Highly diastereoselective solid-phase radical reaction was achieved by using triethylborane and diethylzinc as a radical initiator at low reaction temperature, providing a novel method for the synthesis
Masafumi Ueda
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 124(6), 311-319 (2004-06-01)
The aqueous medium radical reactions of a variety of imine derivatives such as oxime ether, oxime, hydrazone, nitrone, and N-sulfonylimine were investigated. Triethylborane-mediated intermolecular alkyl radical addition to glyoxylic oxime ether, oxime, and nitrone in water proceeded smoothly to give
Ken-ichi Yamada et al.
The Journal of organic chemistry, 77(3), 1547-1553 (2012-01-03)
Triethylborane-mediated tin-free radical alkylation of N-alkoxycarbonyl-imines, such as N-Boc-, N-Cbz-, and N-Teoc-imines, proceeded smoothly at a low temperature (-78 to -20 °C) to give the corresponding adducts in high yield. Although the formation of isocyanate was the major unfavorable reaction
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務