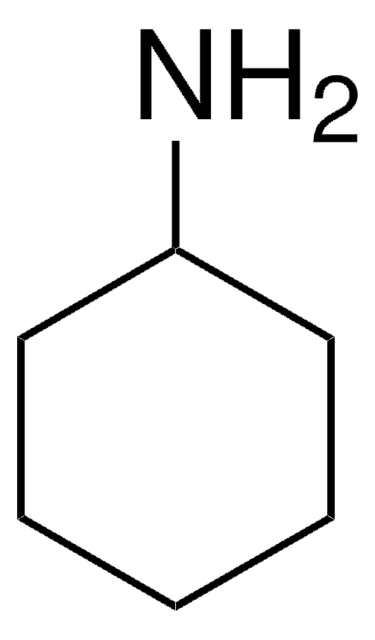
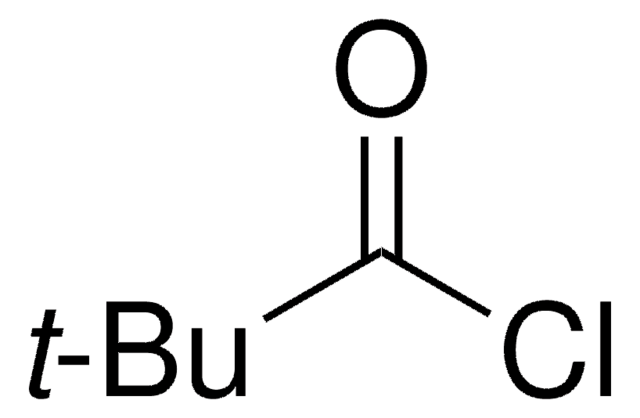
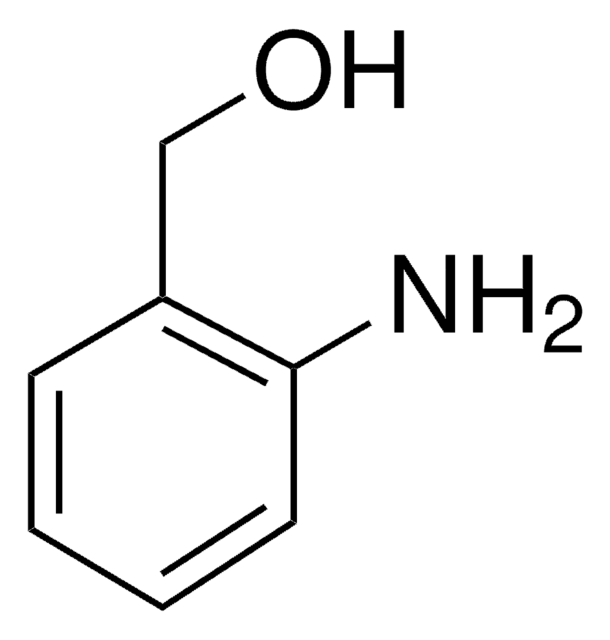
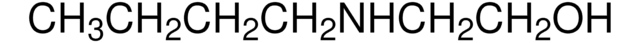推薦產品
蒸汽密度
>1 (vs air)
蒸汽壓力
<0.01 mmHg ( 20 °C)
化驗
98%
形狀
liquid
折射率
n20/D 1.578 (lit.)
bp
278-282 °C/760 mmHg (lit.)
密度
1.094 g/mL at 25 °C (lit.)
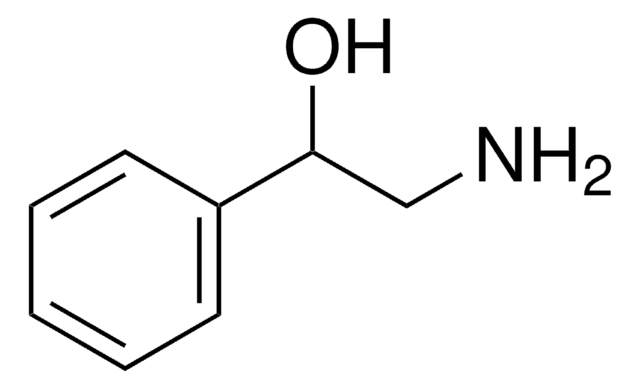
SMILES 字串
OCCNc1ccccc1
InChI
1S/C8H11NO/c10-7-6-9-8-4-2-1-3-5-8/h1-5,9-10H,6-7H2
InChI 密鑰
MWGATWIBSKHFMR-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
N-(2-羟乙基)苯胺被用作人嗅觉UDP-葡糖醛酸糖基转移酶的底物。
訊號詞
Danger
危險分類
Acute Tox. 2 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - STOT RE 2 - STOT SE 1
標靶器官
Blood, Blood,hematopoietic system
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Mercedes Amat et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(28), 7724-7732 (2011-06-15)
Phenylglycinol-derived, unsaturated oxazolopiperidone lactams are extremely useful building blocks that undergo stereoselective conjugate addition reactions with organocuprates, enolates, and sulfur-stabilized anions, allowing the stereocontrolled introduction of substituents at the piperidine 4-position. The factors governing the exo- or endo-facial selectivity are
Alok K Awasthi et al.
The Journal of organic chemistry, 70(14), 5387-5397 (2005-07-02)
[reaction: see text] A practical, large-scale synthesis of a beta-amino ester 1 was developed. A chiral imine derived from (S)-phenylglycinol and 3-trimethylsilylpropanal was coupled with the Reformatsky reagent 3 with high diastereoselectivity (de > 98%) to give (SS)-4a as the
Katarina Babić et al.
Journal of chromatography. A, 1142(1), 84-92 (2006-10-19)
The performance of extractant impregnated resin (EIR) technology for chiral separation of amino-alcohols has been investigated. Phenylglycinol was selected as an archetype model enantiomer and azophenolic crown ether was used as a versatile enantioselective extractant. 1-Phenyloctane was selected as a
Mercedes Amat et al.
Chemical communications (Cambridge, England), (20)(20), 2935-2937 (2009-05-14)
The first total synthesis of (-)-16-episilicine has been completed from a phenylglycinol-derived bicyclic lactam, the key steps being stereoselective conjugate addition and alkylation reactions, and a ring-closing metathesis.
Marco Santarem et al.
The Journal of organic chemistry, 73(16), 6466-6469 (2008-07-22)
An efficient formal total synthesis of (+)-gephyrotoxin is described. The key step of our strategy relies on the diastereoselective reduction of a chiral pyrrolidine beta-enamino ester obtained by condensation of ( S)-phenylglycinol on a protected 8-hydroxy-3,6-dioxooctanoate.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務