肽合成
多肽由两个或多个通过酰胺键连接的氨基酸组成,形成通常包含2至70个氨基酸的氨基酸链。多肽与蛋白质的区别在于,其不需要进行折叠即可获得生物学活性。肽激素(例如血管紧张素、LHRH、脑啡肽)为多肽的内源性形式,在植物和动物中多肽能够以毒素形式存在。多肽可作为药物发现的先导化合物,并且其本身具有药物活性,因而受到广泛关注。此外,它们还可应用于疫苗、生物材料、组织学探针中,并已大量用作产生抗体的抗原。
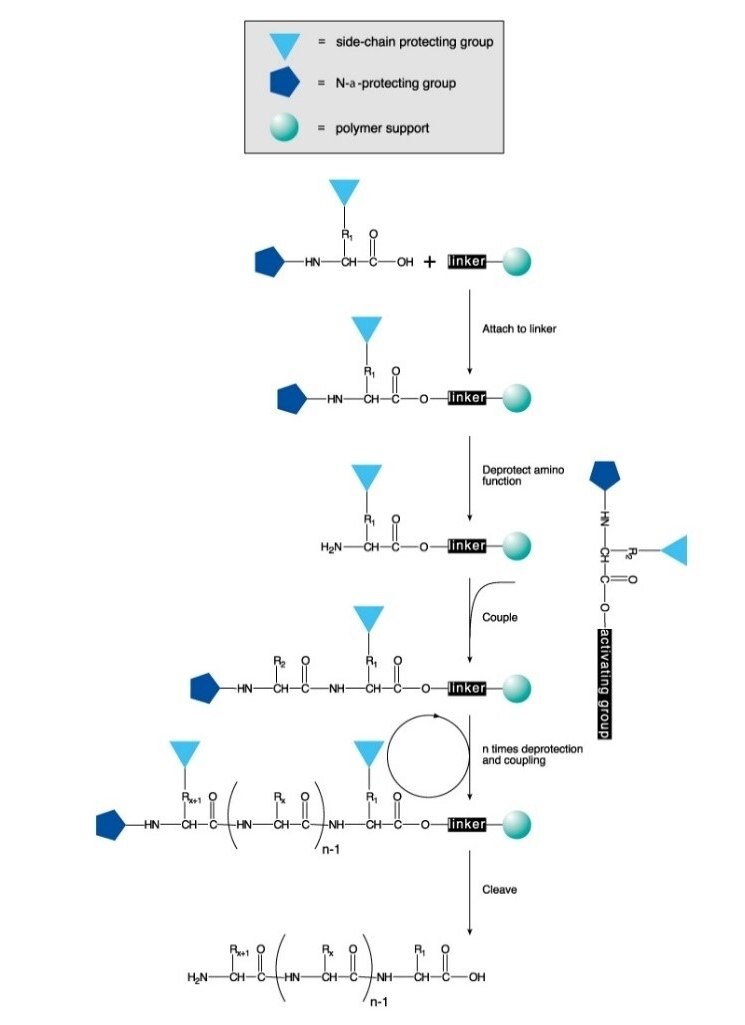
多肽可在溶液中或在固定相上通过化学合成。该过程会在N-保护的氨基酸与带有游离氨基和受保护羧酸的氨基酸之间定向和选择性地形成酰胺键。在固相合成中,羧基保护基团会连接至聚合物载体上。化学键形成后,二肽的氨基保护基将会被去除,同时下一个N-保护的氨基酸则会进行偶联。
相关技术文章
- Fmoc resin cleavage and deprotection follows the difficult task of detaching the peptide from the resin support and removing all the side-chain protecting groups of the amino acid residues to yield the desired peptide.
- Novabiochem® offers a large number of coupling reagents for in situ activation. In situ activating reagents are easy to use, fast reacting – even with sterically hindered amino acids, and their use is generally free of side reactions.
- Review methods and resins for attaching amino acids and peptides, including Merrifield, trityl-based, and hydroxymethyl-functionalized resins. Resin-immobilized peptides can be used for various downstream applications.
- COMU is a non-explosive coupling agent suitable for solution phase & solid phase peptide synthesis. Its activity meets or exceeds that of HATU and its water-soluble by-product are easily removed.
- In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
- 查看完整内容 (22)
相关实验方案
- A guide to create solvent systems used for the thin-layer chromatography assay of Novabiochem products.
- Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
- Amide Coupling in a Box
- Information on the Amide bond and the Catalytic Amide Bond Formation Protocol. Amidation of amines and alcohols. The amide bond, an important linkage in organic chemistry, is a key functional group in peptides, polymers, and many natural products and pharmaceuticals.
- We provide an overview of our available reagents, together with recommendations and details of their use for synthesis of peptides containing post-translationally modified amino acids.
- 查看完整内容 (10)

图 2用于Boc固相肽合成(SPPS)的侧链保护基
由于固相肽合成(SSPS)的效率高、操作简便、速度快、易于并行,因此成为了一种最常用的肽合成方法。SPPS过程涉及将氨基和侧链保护的氨基酸残基顺序添加到与不溶性聚合物支持物相连的氨基酸或肽上(图1)。
酸不稳定的Boc基团(Boc SPPS)或碱不稳定的Fmoc-基团(Fmoc SPPS)已被用于N-α保护。除去该保护基后,下一个被保护氨基酸可通过偶联剂或预活化的被保护氨基酸衍生物进行添加。C-末端氨基酸可通过接头固定于树脂上,该接头的性质决定了链延长后从载体上释放肽所需的条件。通常选择侧链保护基团,以便在将肽从树脂上分离的同时被切割(图2 和 3)。

图 3.用于Fmoc固相肽合成(SPPS)的侧链保护基
大多数多肽通过Fmoc方法制备,最终切割和脱保护通过三氟乙酸处理实现,而Boc方法需要在专业设备中使用剧毒、腐蚀性液体无水HF。
尽管见诸报道的通常是超过100个氨基酸的蛋白质,但此方法也可制备常规50个氨基酸的多肽。通过溶液中完全脱保护的多肽的天然化学连接来反应,制备更长的蛋白质。这种方法可合成难以在细菌中表达的天然肽,掺入非天然或D-氨基酸,生成环状、带支链、标记、以及带有翻译后修饰的多肽。
除了现有工业大规模肽合成工艺,Boc或Z-氨基保护液相肽合成方法已被固相肽合成取代。
若要繼續閱讀,請登入或建立帳戶。
還沒有帳戶?