推荐产品
生物源
Manihot esculenta root (Cassava)
品質等級
化驗
≥99% (HPLC)
形狀
powder
光學活性
[α]20/D 174 to 186 °, c = 1.0% (w/v) in water
技術
HPLC: suitable
雜質
7.3-11.6% water (Karl Fischer)
顏色
white
mp
97-99 °C
溶解度
water: 50 mg/mL, clear, colorless to very faintly yellow
儲存溫度
room temp
SMILES 字串
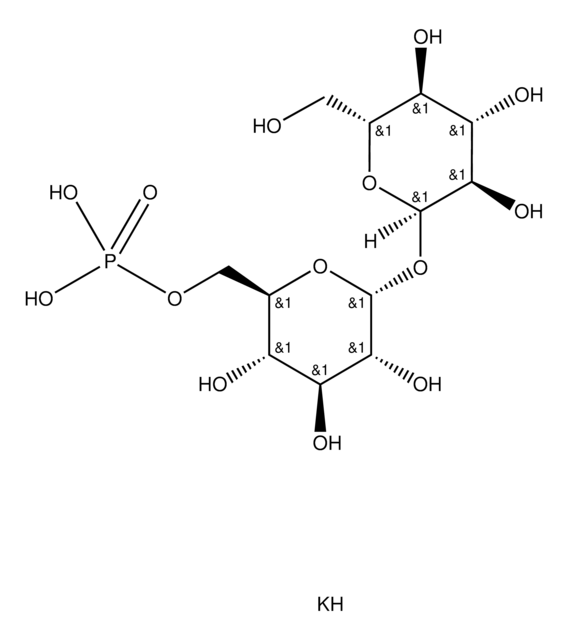
[H]O[H].[H]O[H].OC[C@H]1O[C@H](O[C@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C12H22O11.2H2O/c13-1-3-5(15)7(17)9(19)11(21-3)23-12-10(20)8(18)6(16)4(2-14)22-12;;/h3-20H,1-2H2;2*1H2/t3-,4-,5-,6-,7+,8+,9-,10-,11-,12-;;/m1../s1
InChI 密鑰
DPVHGFAJLZWDOC-PVXXTIHASA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
海藻糖是一种由两个葡萄糖单位通过1-1α键结合而形成的非还原性糖。这种二糖广泛存在于原核生物和真核生物等微生物中。
應用
D-(+)-海藻糖二水化物已被用于:
- 用于海藻糖测试以稳定药物产品
- 作为海藻糖/蔗糖溶液的成分用于制备J-聚集体(J-aggregate)
- 用于制备模拟蜂蜜糖混合物(SHSC)以研究其对蛋白质化学和热稳定性的影响
生化/生理作用
海藻糖是一种1-α(二糖)糖,它有助于植物和动物抵御长期的脱水。海藻糖可用作各种细胞冷冻培养基中的冷冻保护剂。它是一种主要的碳源,也是一种在应对渗透胁迫和热休克时积累的相容性溶质。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Theresa Cloutier et al.
The journal of physical chemistry. B, 122(40), 9350-9360 (2018-09-15)
The CHARMM36 carbohydrate parameter set did not adequately reproduce experimental thermodynamic data of carbohydrate interactions with water or proteins or carbohydrate self-association; thus, a new nonbonded parameter set for carbohydrates was developed. The parameters were developed to reproduce experimental Kirkwood-Buff
Alan Twomey et al.
International journal of pharmaceutics, 487(1-2), 91-100 (2015-04-19)
In frozen and lyophilized systems, the biological to be stabilized (e.g. therapeutic protein, biomarker, drug-delivery vesicle) and the cryo-/lyo-protectant should be co-localized for successful stabilization. During freezing and drying, many factors cause physical separation of the biological from the cryo-/lyo-protectant
Sabine Ullrich et al.
Journal of pharmaceutical sciences, 104(6), 2040-2046 (2015-04-03)
The importance of cake adhesion to the inside vial wall during lyophilization of amorphous trehalose cakes was determined by using hydrophobized vials. The degrees of cake shrinkage and cracking were determined independently by photographic imaging of the cake top surface
Joachim Schaefer et al.
International journal of pharmaceutics, 489(1-2), 124-130 (2015-05-06)
The inactivation of catalase during spray-drying over a range of outlet gas temperatures could be closely represented by the Arrhenius equation. From this an activation energy for damage to the catalase could be calculated. The close fit to Arrhenius suggests
Anke Sass et al.
Drug development and industrial pharmacy, 40(6), 749-757 (2013-04-20)
The spray-drying behaviour of 16 water-miscible organic solvents on a bench-scale machine (Büchi B290 with inert loop) was determined under mild-to-moderate process conditions, namely inlet gas temperature of 130 °C and liquid feed flow rate of ≤3 mL/min. The solvents with boiling
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门