推荐产品
product name
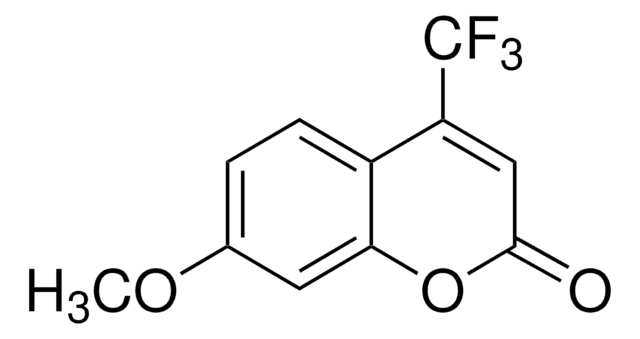
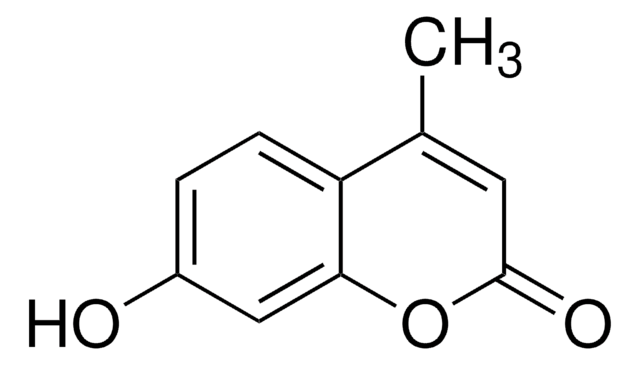
7-Ethoxy-4-(trifluoromethyl)coumarin, ≥98% (TLC)
化驗
≥98% (TLC)
形狀
powder
溶解度
chloroform: 100 mg/mL, clear, colorless (Soluble in chloroform, methanol, and DMSO.)
螢光
λex 333 nm; λem 415 nm in methanol
儲存溫度
−20°C
SMILES 字串
CCOc1ccc2c(OC(=O)C=C2C(F)(F)F)c1
InChI
1S/C12H9F3O3/c1-2-17-7-3-4-8-9(12(13,14)15)6-11(16)18-10(8)5-7/h3-6H,2H2,1H3
InChI 密鑰
OLHOIERZAZMHGK-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
特點和優勢
7-Ethoxy-4-(trifluoromethyl)coumarin is a fluorogenic substrate for cytochrome P-450 catalyzed O-de-ethylation.
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
J G DeLuca et al.
Biochemical pharmacology, 37(9), 1731-1739 (1988-05-01)
The microsomal O-deethylation of a novel coumarin analog, 7-ethoxy-4-trifluoromethylcoumarin (EFC), to a fluorescent product was characterized. Results indicate that this analog provides a rapid, convenient and highly sensitive means to assay cytochrome P-450-mediated metabolism. Like microsomal 7-ethoxycoumarin (7-EC) O-deethylation, EFC
S Löfgren et al.
Xenobiotica; the fate of foreign compounds in biological systems, 34(9), 811-834 (2005-03-04)
The aim was to characterize mouse gender and strain differences in the metabolism of commonly used human cytochrome (CYP) P450 probe substrates. Thirteen human CYP probe substrates (phenacetin, coumarin, 7-ethoxy-4-trifluoromethyl coumarin, amiodarone, paclitaxel, diclofenac, S-mephenytoin, bufuralol, dextromethorphan, chlorzoxazone, p-nitrophenol, testosterone
E S Roberts et al.
Chemical research in toxicology, 11(9), 1067-1074 (1998-10-07)
The addition of peroxynitrite to purified cytochrome P450 2B1 resulted in a concentration-dependent loss of the NADPH- and reductase-supported or tert-butylhydroperoxide-supported 7-ethoxy-4-(trifluoromethyl)coumarin O-deethylation activity of P450 2B1 with IC50 values of 39 and 210 microM, respectively. After incubation of P450
Thomas Van Leeuwen et al.
Pest management science, 62(5), 425-433 (2006-03-22)
Tetranychus urticae Koch has recently developed resistance to chlorfenapyr in Australia and Japan, but no attempt has yet been made to describe the biochemical mechanisms involved in chlorfenapyr resistance. In this study a laboratory-selected chlorfenapyr-resistant strain was investigated. Resistance to
Maori Mitsuda et al.
Protein expression and purification, 46(2), 401-405 (2005-11-29)
Improvement of CYP2B6 expression was examined by co-expression with molecular chaperones GroES/EL. Although a CO-reduced difference spectrum was not detected in Escherichia coli transformed only by the CYP2B6-expressing vector, co-expression of GroES/EL resulted in high-level expression which reached over 2000
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门