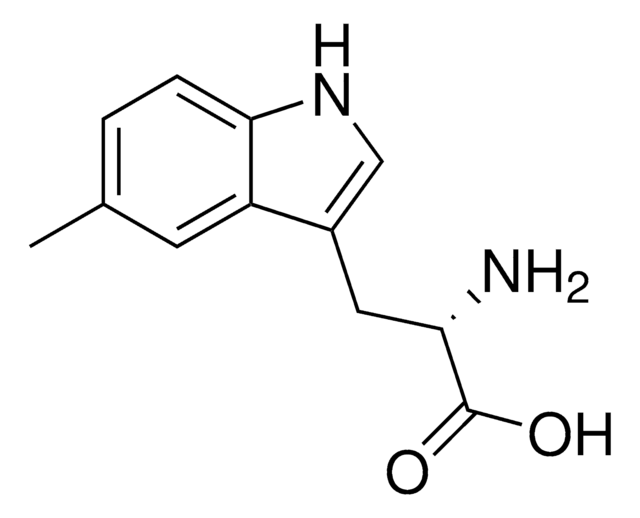
推荐产品
品質等級
化驗
≥97% (TLC)
形狀
powder
顏色
white to faint yellow
mp
280-282 °C
應用
peptide synthesis
儲存溫度
2-8°C
SMILES 字串
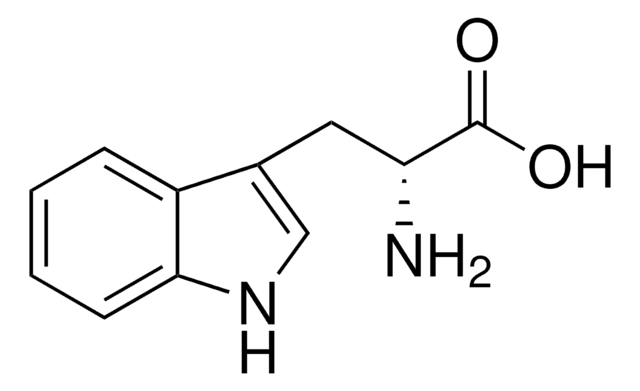
Cc1ccc2[nH]cc(CC(N)C(O)=O)c2c1
InChI
1S/C12H14N2O2/c1-7-2-3-11-9(4-7)8(6-14-11)5-10(13)12(15)16/h2-4,6,10,14H,5,13H2,1H3,(H,15,16)
InChI 密鑰
HUNCSWANZMJLPM-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
- 通过调节肠道菌群缓解结肠炎:代谢产物5-甲基-ʟ-色氨酸(5-MT)来自当归多糖,可通过调节肠道菌群和TLR4/MyD88/NF-κB信号通路缓解结肠炎。说明5-MT在炎症性肠病研究和治疗中的应用前景(Zou et al., 2023)。
- 色氨酸衍生物的酶促合成:研究由氨基酸通过一锅对映选择性合成天然(S)-spirobrassinin和非天然(S)-甲基spirobrassinin,使用芜菁酶。介绍一种色氨酸衍生物的新合成方法,适合用于生化分析(Ryu et al., 2021)。
生化/生理作用
5-甲基- DL -色氨酸抑制邻氨基苯甲酸酯化合物的合成,该化合物是粗糙脉孢菌 色氨酸生物合成的第一步 。5-甲基- DL -色氨酸是大肠杆菌色氨酸阻抑物的辅阻遏子。
5-甲基-DL-色氨酸可用于选择 产甲烷菌 (原始细菌)PS 株的基因突变株。5-甲基色氨酸是一种抑制色氨酸操纵子表达。5-甲基色氨酸是色氨酸酶的底物。5-甲基色氨酸抑制燕麦中激发子对邻氨基苯甲酸合成酶活性的诱导。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
X H Zhang et al.
Plant physiology, 127(1), 131-141 (2001-09-13)
Anthranilate synthase (AS), the control enzyme of the tryptophan (Trp) biosynthetic pathway, is encoded by nuclear genes, but is transported into the plastids. A tobacco (Nicotiana tabacum) cDNA (ASA2) encoding a feedback-insensitive tobacco AS alpha-subunit was transformed into two different
G Lester
Journal of bacteriology, 96(5), 1768-1773 (1968-11-01)
The in vivo regulation of intermediate reactions in the pathway of tryptophan synthesis in Neurospora crassa was examined in a double mutant (tr-2, tr-3) which lacks the functions of the first and last enzymes in the pathway from chorismic acid
Vered Tzin et al.
The New phytologist, 194(2), 430-439 (2012-02-03)
The shikimate pathway of plants mediates the conversion of primary carbon metabolites via chorismate into the three aromatic amino acids and to numerous secondary metabolites derived from them. However, the regulation of the shikimate pathway is still far from being
Tetsuya Matsukawa et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 57(1-2), 121-128 (2002-04-03)
Oat phytoalexins, avenanthramides, are a series of substituted hydroxycinnamic acid amides with anthranilate. The anthranilate in avenanthramides is biosynthesized by anthranilate synthase (AS, EC 4.1.3.27). Induction of anthranilate synthase activity was investigated in oat leaves treated with oligo-N-acetylchitooligosaccharide elicitors. AS
E I Hyde et al.
European journal of biochemistry, 201(3), 569-579 (1991-11-01)
The Escherichia coli trp repressor binds to the trp operator in the presence of tryptophan, thereby inhibiting tryptophan biosynthesis. Tryptophan analogues lacking the alpha-amino group act as inducers of trp operon expression. We have used one- and two-dimensional 1H-NMR spectroscopy
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门