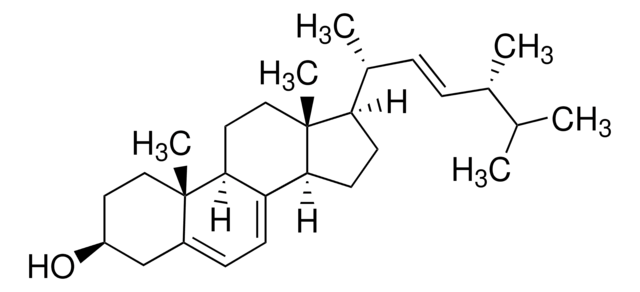
推荐产品
生物源
microbial
品質等級
化驗
≥75%
形狀
powder
顏色
white to off-white
mp
156-158 °C (lit.)
儲存溫度
2-8°C
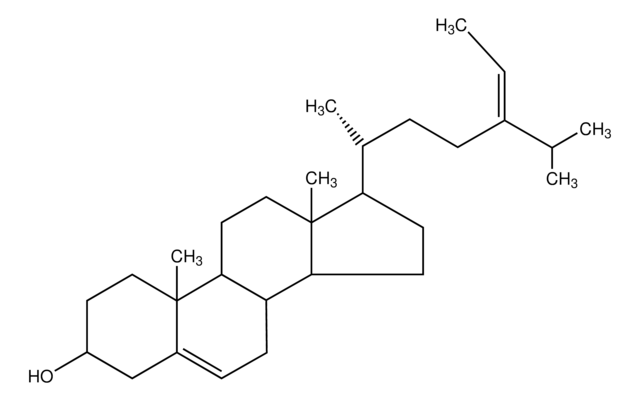
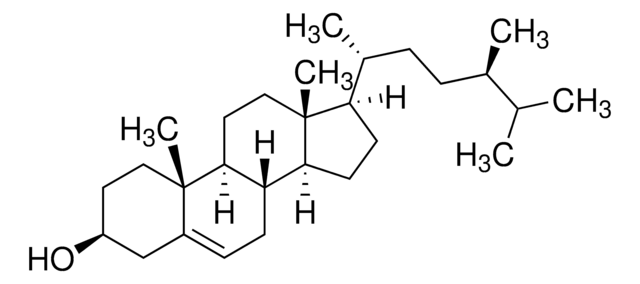
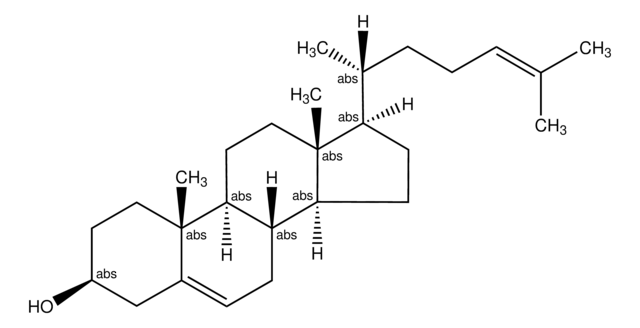
SMILES 字串
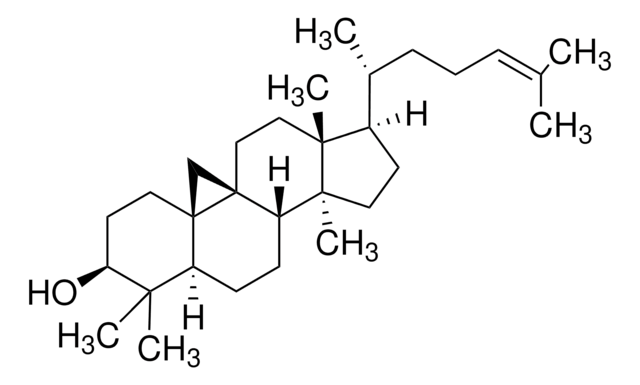
[H][C@@]1(CC[C@@]2([H])C3=CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)\C=C\[C@H](C)C(C)C
InChI
1S/C28H44O/c1-18(2)19(3)7-8-20(4)24-11-12-25-23-10-9-21-17-22(29)13-15-27(21,5)26(23)14-16-28(24,25)6/h7-10,18-20,22,24-26,29H,11-17H2,1-6H3/b8-7+/t19-,20+,22-,24+,25-,26-,27-,28+/m0/s1
InChI 密鑰
DNVPQKQSNYMLRS-APGDWVJJSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- 作为酵母细胞单层模型的成分用于研究类固醇和三萜皂苷对单层的作用。
- 作为标准样品用于分离和鉴定含麦角固醇的真菌代谢物成分。
- 作为培养基成分用于分离布氏冈比亚锥虫的CYP51RNAi 表型,以验证麦角固醇的生物合成
生化/生理作用
其他客户在看
商品
Vitamin D2 (ergocalciferol) is naturally synthesized from ergosterol by invertebrates, fungi, and plants in response to ultraviolet B irradiation, while vitamin D3 synthesis (cholecalciferol) is uniquely initiated in the skin of vertebrates. During sun exposure, ultraviolet B photons are absorbed by 7-dehydrocholesterol, which is found within the plasma membranes of epidermal and dermal skin layers. This reaction yields an unstable derivative of 7-dehydrocholesterol, named precholecalcitrol, which rapidly rearranges to vitamin D3. Vitamin D binding protein (DBP) is a carrier protein responsible for drawing vitamin D3 from the plasma membrane into the dermal capillaries within the extracellular space.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门