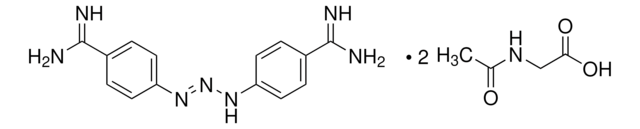
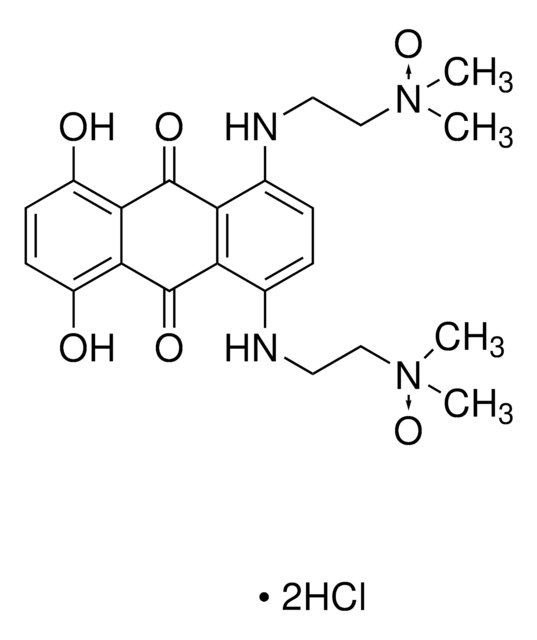
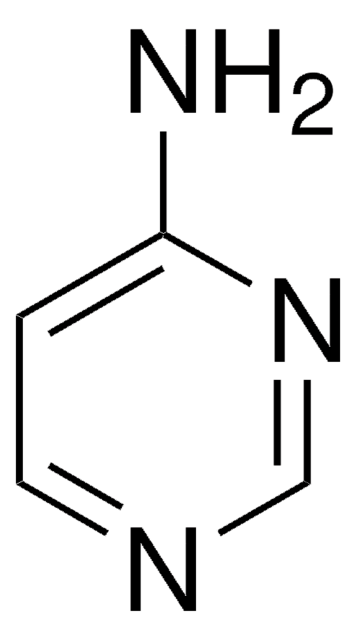
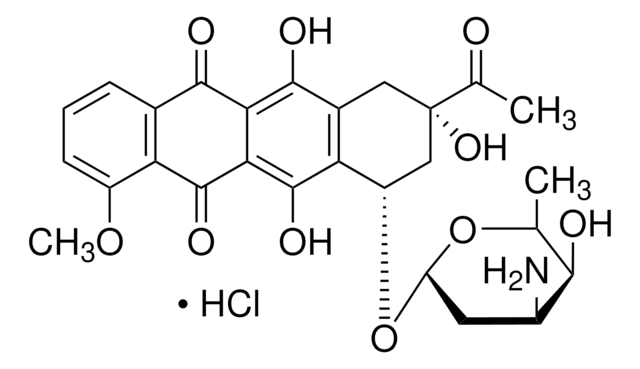
推荐产品
品質等級
化驗
≥98% (HPLC)
形狀
solid
儲存條件
desiccated
溶解度
deionized water: 8 mg/mL
儲存溫度
room temp
SMILES 字串
Cl.Cl.C1CN=C(N1)N\N=C\c2c3ccccc3c(\C=N\NC4=NCCN4)c5ccccc25
InChI
1S/C22H22N8.2ClH/c1-2-6-16-15(5-1)19(13-27-29-21-23-9-10-24-21)17-7-3-4-8-18(17)20(16)14-28-30-22-25-11-12-26-22;;/h1-8,13-14H,9-12H2,(H2,23,24,29)(H2,25,26,30);2*1H/b27-13+,28-14+;;
InChI 密鑰
KINULKKPVJYRON-PVNXHVEDSA-N
應用
Bisantrene (NSC 337766), and its derivatives are topoisomerase II poisons and DNA intercalators. It may be used as model compounds to study P-glycoprotein-mediated multiple drug resistance (MDR1). Bisantrene may be used as a Rac1 inhibitor.
生化/生理作用
Bisantrene dihydrochloride is an antineoplastic agent, MDR1 substrate, DNA intercalator, and topoisomerase II poison. Cancer cells that develop resistance to bisantrene tend to overexpress P-glycoprotein. Bisantrene can be used to select for P-glycoprotein-mediated multiple drug resistance. The data suggest that bisantrene is an excellent substrate for P-glycoprotein.
Bisantrene is an anthracene bishydrazone derivative, which has antitumor activity.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
G Capranico et al.
Journal of molecular biology, 235(4), 1218-1230 (1994-01-28)
To gain further knowledge of the molecular features of topoisomerase II inhibitors required for drug-receptor complex formation, we investigated the conformational drug determinants of the sequence specificities of drug-stimulated DNA cleavage by computer-aided molecular modeling techniques. DNA sequence specificities of
T P Wunz et al.
Journal of medicinal chemistry, 33(6), 1549-1553 (1990-06-01)
The relative DNA binding strengths of bisantrene and nine new analogues were measured by spectrophotometric titration and melt transition temperature (Tm) techniques. Data from the spectrophotometric titrations could not be fit by simple Scatchard plots. However, they were fit by
K C Murdock et al.
Journal of medicinal chemistry, 36(15), 2098-2101 (1993-07-23)
The selective phosphorylation of bisantrene (1) affords bis(phosphonoguanidinic acid) 6, a prodrug with enhanced aqueous solubility (as sodium salt 7) at physiological pH. Unlike 1, in a rat tail vein model, no precipitation was observed when bis(phosphonoguanidinic acid) 6 was
A Spadea et al.
Leukemia & lymphoma, 9(3), 217-220 (1993-02-01)
Because of the lack of standard treatment in refractory and relapsed acute myelogenous leukemia (AML) several new drugs have been employed alone to evaluate their efficacy in this peculiar category of patient. Bisantrene, a new anthracene bishydrazone derivative, has shown
G Visani et al.
Haematologica, 75(6), 527-531 (1990-11-01)
In vitro clonogenic assays may be useful for determining the sensitivity of leukemic cells to chemotherapeutic agents. We evaluated the antileukemic effect of Bisantrene (an anthracene derivative now undergoing phase II clinical trials in relapsed/resistant acute non lymphoid leukemias-ANLL) using
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门