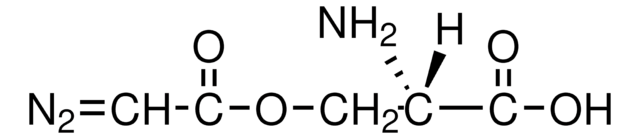推荐产品
品質等級
化驗
≥98% (TLC)
形狀
powder
顏色
off-white to yellow-green
抗生素活性譜
fungi
作用方式
enzyme | inhibits
儲存溫度
−20°C
SMILES 字串
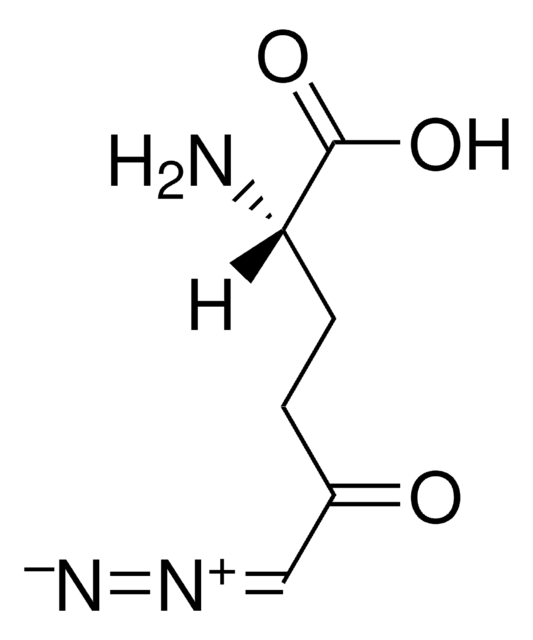
N[C@@H](COC(=O)C=[N+]=[N-])C(O)=O
InChI
1S/C5H7N3O4/c6-3(5(10)11)2-12-4(9)1-8-7/h1,3H,2,6H2,(H,10,11)/t3-/m0/s1
InChI 密鑰
MZZGOOYMKKIOOX-VKHMYHEASA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Chemical structure: amino acid derivatives
應用
Used in cell culture for the selection of HGPRT revertants.
生化/生理作用
Azaserine is an antibiotic and antifungal; it may also act as a tumor inducer. It is a structural analog of glutamine and competes with glutamine in binding to enzymes involved in purine biosynthesis. Azaserine inhibits purine biosynthesis by covalently reacting with cysteine residues in the enzyme active sites, such as in formylglycinamide ribonucleotide amidotransferase and PRPP amidotransferase. Azaserine can induce DNA damage via the formation of carboxymethylated bases and O6-methylguanine. Secretion of exo-1,3-β-glucanase and germ-tube formation of Candida albicans were inhibited by azaserine.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Carc. 2
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Timea Beleznai et al.
Vascular pharmacology, 56(3-4), 115-121 (2011-12-14)
We hypothesized that under high glucose conditions, activation of the hexosamine pathway leads to impaired nitric oxide (NO)-dependent arteriolar dilation. Skeletal muscle arterioles (diameter: ~160μm) isolated from male Wistar rats were exposed to normal glucose (NG, 5.5mmol/L) or high glucose
S P Ram et al.
Journal of general microbiology, 130(5), 1227-1236 (1984-05-01)
Exo-(1----3)-beta-glucanase, beta-glucosidase, autolysin and trehalase were assayed in situ in Candida albicans during yeast growth, starvation and germ-tube formation. Cell viability, germ-tube formation, intracellular glucose-6-phosphate dehydrogenase and beta-glucosidase were unaffected in cells incubated in 0.1 M-HC1 for 15 min at
Kornberg, A., and Baker, T.
DNA Replication, 57-60 (1992)
Tusty-Jiuan Hsieh et al.
The Journal of endocrinology, 183(3), 535-550 (2004-12-14)
We reported previously that insulin inhibits the stimulatory effect of high glucose on the expression of angiotensinogen (ANG) gene in both rat immortalized renal proximal tubular cells (IRPTCs) and non-diabetic rat renal proximal tubular cells (RPTCs), but has no effect
Angana Gupta Rajapakse et al.
American journal of physiology. Heart and circulatory physiology, 296(3), H815-H822 (2009-01-13)
Hexosamine biosynthetic pathway (HBP) accounts for some cardiovascular adverse effects of hyperglycemia. We investigated whether the HBP inhibitor azaserine protects against hyperglycemia-induced endothelial damage dependently of HBP. Human endothelial cells isolated from umbilical veins were exposed either to a high
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门