所有图片(1)
About This Item
经验公式(希尔记法):
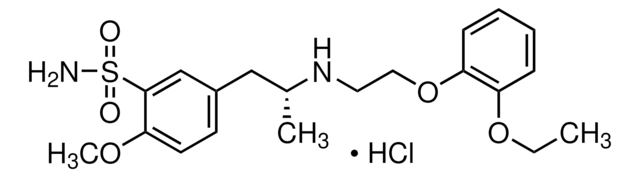
C20H28N2O5S · HCl
CAS号:
分子量:
444.97
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to BP 876
traceable to Ph. Eur. Y0000650
traceable to USP 1643260
API 家族
tamsulosin
CofA
current certificate can be downloaded
包裝
ampule of 1 × 200 mg
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
-10 to -25°C
SMILES 字串
Cl.CCOc1ccccc1OCCN[C@H](C)Cc2ccc(OC)c(c2)S(N)(=O)=O
InChI
1S/C20H28N2O5S.ClH/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24;/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24);1H/t15-;/m1./s1
InChI 密鑰
ZZIZZTHXZRDOFM-XFULWGLBSA-N
基因資訊
human ... ADRA1A(148) , ADRA1B(147) , ADRA1D(146)
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
Tamsulosin hydrochloride is a subtypeselective a1A and a1D adrenoceptor antagonist, which exists in two enantiomeric forms, of which the R-isomer is the pharmaceutically active component. It is used to reduce urinary obstruction and is also involved in relieving the symptoms associated with symptomatic benign prostatic hyperplasia.
應用
Tamsulosin hydrochloride may be used as a pharmaceutical reference standard for the quantification of the analyte in pharmaceutical formulations using various chromatography techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
生化/生理作用
Tamsulosin is a α1A/1D-adrenoceptor antagonist; Used as a treatment of BPH (benign prostatic hypertrophy).
Tamsulosin is an α1A/1D-adrenoceptor antagonist used as a treatment of benign prostatic hypertrophy (BPH). Its activity as an ? blocker also affects the iris, and has led to complications during cataract surgery, a condition called "floppy iris" syndrome.
腳註
To see an example of a Certificate of Analysis for this material enter LRAC0288 in the slot below. This is an example certificate only and may not be the lot that you receive.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Validated stability indicating HPTLC method for the determination of Tamsulosin hydrochloride in pharmaceutical dosage forms
Choudhari.PV and Nikalje GPA
International Journal of ChemTech Research, 2(1), 646-652 (2010)
Development and validation of stability-indicating HPTLC determination of tamsulosin in bulk and pharmaceutical dosage form
Bari.BS, et al.
Chromatography Research International, 2011(2), 197-205 (2011)
Validated RP-HPLC and TLC methods for simultaneous estimation of tamsulosin hydrochloride and finasteride in combined dosage forms
Patel B.D and Patel J.N
Acta pharmaceutica, 60(2), 197-205 (2010)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门