推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. F0048000
traceable to USP 1269447
API 家族
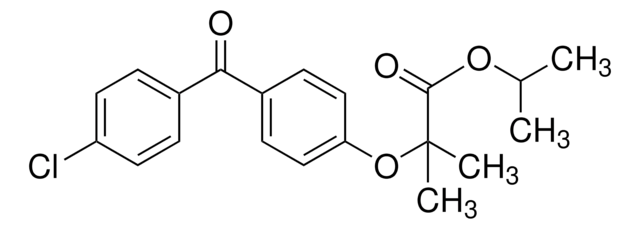
fenofibrate
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
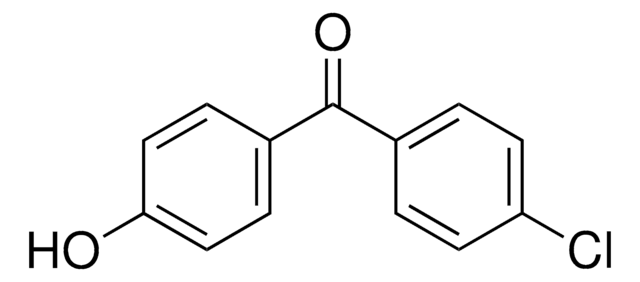
SMILES 字串
CC(C)OC(=O)C(C)(C)Oc1ccc(cc1)C(=O)c2ccc(Cl)cc2
InChI
1S/C20H21ClO4/c1-13(2)24-19(23)20(3,4)25-17-11-7-15(8-12-17)18(22)14-5-9-16(21)10-6-14/h5-13H,1-4H3
InChI 密鑰
YMTINGFKWWXKFG-UHFFFAOYSA-N
基因資訊
human ... PPARA(5465)
正在寻找类似产品? 访问 产品对比指南
一般說明
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Fenofibrate is a third-generation fibric acid derivative which is widely employed for the treatment of atherogenic dyslipidemia, hypertriglyceridemia, mixed dyslipidemia and hypercholesterolemia.
Fenofibrate is a third-generation fibric acid derivative which is widely employed for the treatment of atherogenic dyslipidemia, hypertriglyceridemia, mixed dyslipidemia and hypercholesterolemia.
應用
Fenofibrate may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by various chromatography and spectrophotometry techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAC0257 in the slot below. This is an example certificate only and may not be the lot that you receive.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
STOT RE 2 Oral
標靶器官
Liver
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Simultaneous Estimation of Rosuvastatin Calcium and Fenofibrate in Bulk and in Tablet Dosage Form by UV-Spectrophotometry and RP-HPLC
Karunakaran A, et al.
Stamford Journal of Pharmaceutical Sciences, 4(1), 58-63 (2011)
Estimation of Atorvastatin Calcium and Fenofibrate in Tablets by Derivative Spectrophotometry and Liquid Chromatography
Nakarani NV, et al.
Journal of AOAC (Association of Official Analytical Chemists) International, 90(3), 700-705 (2007)
Electrochemical studies and square-wave voltammetric determination of fenofibrate in pharmaceutical formulations
Yardimci C and Ozaltin N
Analytical and Bioanalytical Chemistry, 378(2), 495-498 (2004)
Stanley M H Chan et al.
Diabetes, 62(6), 2095-2105 (2013-01-26)
Endoplasmic reticulum (ER) stress is suggested to cause hepatic insulin resistance by increasing de novo lipogenesis (DNL) and directly interfering with insulin signaling through the activation of the c-Jun N-terminal kinase (JNK) and IκB kinase (IKK) pathway. The current study
Cordula Stillhart et al.
The Journal of pharmacy and pharmacology, 65(2), 181-192 (2013-01-03)
To advance in vitro screening of surfactant/co-solvent formulations in early development by considering drug supersaturation and the mechanism of solubilization upon aqueous dilution. Two surfactant/co-solvent model systems were studied at practically relevant aqueous dilution ratios. Precipitation of the model drug
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门