推荐产品
等級
pharmaceutical primary standard
API 家族
cinnarizine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
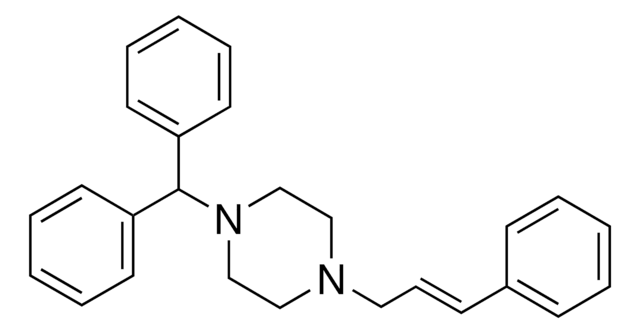
C1CN(CCN1C\C=C\c2ccccc2)C(c3ccccc3)c4ccccc4
InChI
1S/C26H28N2/c1-4-11-23(12-5-1)13-10-18-27-19-21-28(22-20-27)26(24-14-6-2-7-15-24)25-16-8-3-9-17-25/h1-17,26H,18-22H2/b13-10+
InChI 密鑰
DERZBLKQOCDDDZ-JLHYYAGUSA-N
基因資訊
human ... CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779) , HRH1(3269)
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Cinnarizine EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Anne T Larsen et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 48(1-2), 339-350 (2012-11-28)
The in vivo performance of self-nanoemulsifying drug delivery systems (SNEDDSs) with different in vitro physicochemical properties were determined with the purpose of elucidating the parameters determining the in vivo performance of SNEDDSs. The in vitro characterisation included the use of
P Y Cezarino et al.
Climacteric : the journal of the International Menopause Society, 14(4), 492-496 (2011-03-24)
To evaluate the effectiveness and safety of cinnarizine in the treatment of menopausal symptoms. A total of 100 climacteric and symptomatic women participated in a double-blind, placebo-controlled study. They were divided into two groups of the same size: Gcin, intake
Mohamed A Alhnan et al.
International journal of pharmaceutics, 416(1), 55-60 (2011-06-18)
Poorly water soluble basic drugs are very sensitive to pH changes and following dissolution in the acidic stomach environment tend to precipitate upon gastric emptying, which leads to compromised or erratic oral bioavailability. In this work, we show that the
Amnon Gil et al.
Clinical neuropharmacology, 35(1), 37-39 (2011-12-06)
The objective of the study was to compare the efficacy of transdermal scopolamine and cinnarizine in the prevention of seasickness and their adverse reactions. Seventy-six naval crew members participated in a double-blind, randomized, crossover study. On 2 voyages, they were
Tri-Hung Nguyen et al.
Journal of controlled release : official journal of the Controlled Release Society, 153(2), 180-186 (2011-04-19)
This study is the first to demonstrate the ability of nanostructured liquid crystal particles to sustain the absorption of a poorly water soluble drug after oral administration. Cubic (V(2)) liquid crystalline nanostructured particles (cubosomes) formed from phytantriol (PHY) were shown
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门