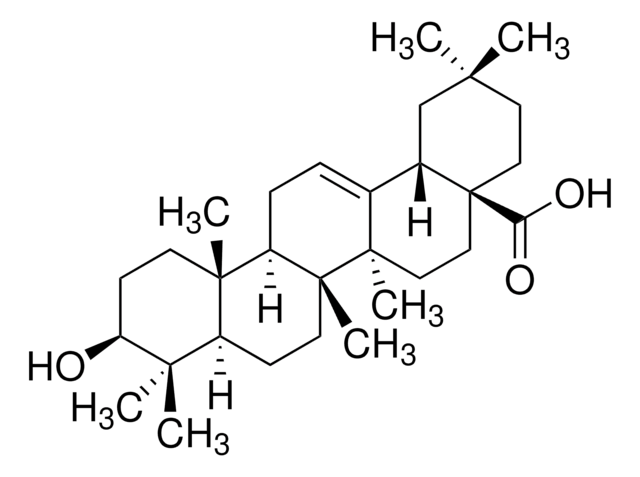
推荐产品
等級
analytical standard
品質等級
化驗
≥98.5% (HPLC)
技術
HPLC: suitable
gas chromatography (GC): suitable
mp
292 °C (dec.) (lit.)
應用
food and beverages
形式
neat
SMILES 字串
[H][C@@]12CC[C@]3(C)[C@]([H])(CC=C4[C@]5([H])[C@@H](C)[C@H](C)CC[C@@]5(CC[C@@]34C)C(O)=O)[C@@]1(C)CC[C@H](O)C2(C)C
InChI
1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1
InChI 密鑰
WCGUUGGRBIKTOS-GPOJBZKASA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
熊果酸属于三萜类化合物,广泛存在于草药、食品和其他植物中。它可能具有药理活性,如心脏保护、镇痛、强心、镇静和滋补作用。
應用
有关合适仪器技术的更多信息,请参考产品′s分析证书。如需进一步支持,请联系技术服务。
生化/生理作用
三萜类化合物存在于苹果等各类水果中,它是一种心脏保护剂和抗肿瘤剂。由于它能够抑制淀粉样蛋白β和CD36受体之间的相互作用,因此在研究中被用作潜在的阿尔茨海默氏病治疗剂。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
580.5 °F
閃點(°C)
304.7 °C
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Pharmacology of oleanolic acid and ursolic acid
Liu J
Journal of Ethnopharmacology, 49, 57-68 (1995)
Jing-Wei Shao et al.
European journal of medicinal chemistry, 46(7), 2652-2661 (2011-04-26)
Twenty-three ursolic acid (1) derivatives 2-24 (ten novel compounds 8-10, 14-17 and 22-24) modified at the C-3 and the C-28 positions were synthesized, and their structures were confirmed by IR, (1)H NMR, MS, and elemental analysis. The single crystals of
Jin-Feng Hu et al.
Journal of natural products, 69(1), 118-120 (2006-01-31)
One new (1) and four known (2-5) ursene triterpenes with potent inhibition of the formation of the bacterial biofilm Pseudomonas aeruginosa PA01 were obtained from Diospyros dendo using a high-throughput natural products chemistry procedure. These compounds were isolated as mass-limited
Huang-Yao Tu et al.
Bioorganic & medicinal chemistry, 17(20), 7265-7274 (2009-09-18)
Twenty-three ursolic acid (1) derivatives 2-24 including nine new 1 derivatives 5, 7-11, 20-22 were synthesized and evaluated for cytotoxicities against NTUB1 cells (human bladder cancer cell line). Compounds 5 and 17 with an isopropyl ester moiety at C-17-COOH and
Ming-Chuan Liu et al.
European journal of medicinal chemistry, 58, 128-135 (2012-11-06)
This study designed and synthesized a series of novel ursolic acid derivatives in an attempt to develop potent antitumor agents. Their structures were confirmed using MS, IR, (1)H NMR and (13)C NMR. The inhibitory activities of the title compounds against
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门