14407
N-(+)-Biotinyl-6-aminohexanoic acid
≥97.0% (HPLC)
别名:
(+)-Biotin-ε-aminocaproic acid, 6-((Biotinoyl)amino)hexanoic acid, Biotin-X, N-(+)-Biotinyl-6-aminocaproic acid
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C16H27N3O4S
CAS号:
分子量:
357.47
Beilstein:
706392
MDL號碼:
分類程式碼代碼:
12352106
PubChem物質ID:
NACRES:
NA.79
推荐产品
品質等級
化驗
≥97.0% (HPLC)
形狀
powder
顏色
colorless to white
mp
220-230 °C (dec.)
正離子痕跡
Ca: ≤20 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
K: ≤50 mg/kg
Mg: ≤5 mg/kg
Mn: ≤5 mg/kg
Na: ≤100 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Zn: ≤5 mg/kg
應用
detection
SMILES 字串
[H][C@]12CS[C@@H](CCCCC(=O)NCCCCCC(O)=O)[C@@]1([H])NC(=O)N2
InChI
1S/C16H27N3O4S/c20-13(17-9-5-1-2-8-14(21)22)7-4-3-6-12-15-11(10-24-12)18-16(23)19-15/h11-12,15H,1-10H2,(H,17,20)(H,21,22)(H2,18,19,23)/t11-,12-,15-/m0/s1
InChI 密鑰
CMUGHZFPFWNUQT-HUBLWGQQSA-N
正在寻找类似产品? 访问 产品对比指南
應用
N-(+)-Biotinyl-6-aminohexanoic acid is suitable to perform biotinylation.
Biotinylation reagent
生化/生理作用
Biotinyl-6-aminohexanoic acid (Biotin-X) may be used in the biosynthesis of biotinylated oligosaccharides. Biotinyl-6-aminohexanoic acid is frequently used as a derivative of N-hydroxysuccinimide ester.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Effective chemoenzymatic synthesis of p-aminophenyl glycosides of sialyl N-acetyllactosaminide and analysis of their interactions with lectins
Zeng X, et al.
Carbohydrate Research, 342(9), 1244-1248 (2007)
C R Valeri et al.
Vox sanguinis, 88(2), 122-129 (2005-02-22)
In accordance with Food and Drug Administration (FDA) regulations, platelets can be stored in the liquid state at 22 degrees C for only 5 days. Platelets frozen with 6% dimethylsulphoxide (DMSO) can be stored at -80 degrees C for 2
Multivalent foldamer-based affinity assay for selective recognition of A beta oligomers
Olajos G, et al.
Analytica Chimica Acta, 960, 131-137 (2017)
Infrared analysis of biomolecule attachment to functionalized silicon surfaces
Biointerface Characterization by Advanced IR Spectroscopy, 83-113 (2011)
Xiaoxiong Zeng et al.
Carbohydrate research, 342(9), 1244-1248 (2007-04-05)
A convenient chemoenzymatic procedure for the synthesis of p-aminophenyl glycosides of sialyl N-acetyllactosaminide has been developed from p-nitrophenyl N-acetyl-beta-D-glucosaminide as starting material through three steps: synthesis of p-nitrophenyl N-acetyllactosaminide with beta-D-galactosidase, chemical reduction of the p-nitrophenyl group, and sialylation with
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门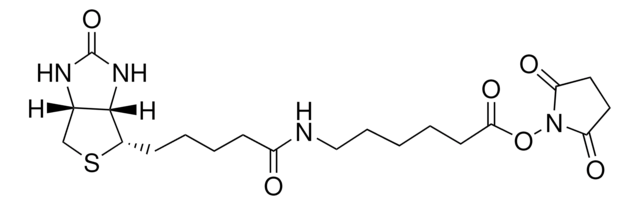


![Fmoc-Thr[GalNAc(Ac)3-α-D]-OH 97%](/deepweb/assets/sigmaaldrich/product/structures/250/325/42734f24-7836-4aaa-ba6a-6284bf7e8181/640/42734f24-7836-4aaa-ba6a-6284bf7e8181.png)