推荐产品
形狀
liquid
反應適用性
reaction type: C-C Bond Formation
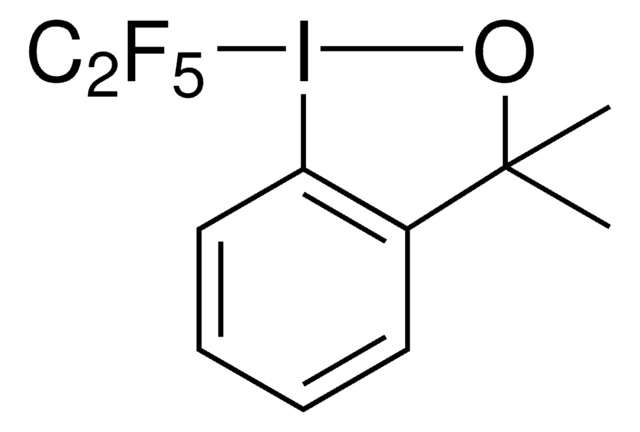
SMILES 字串
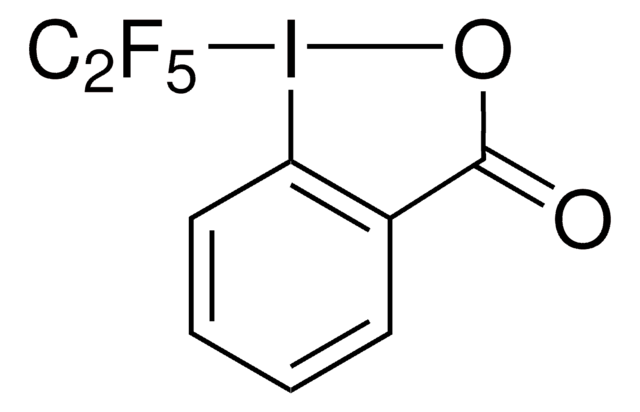
FC(F)(Sc1ccccc1)C(F)(F)Br
InChI
1S/C8H5BrF4S/c9-7(10,11)8(12,13)14-6-4-2-1-3-5-6/h1-5H
InChI 密鑰
ACKRNLPIVVTRML-UHFFFAOYSA-N
應用
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
法律資訊
訊號詞
Warning
危險聲明
危險分類
Aquatic Chronic 4 - Eye Irrit. 2
儲存類別代碼
10 - Combustible liquids
閃點(°F)
Not applicable
閃點(°C)
Not applicable
商品
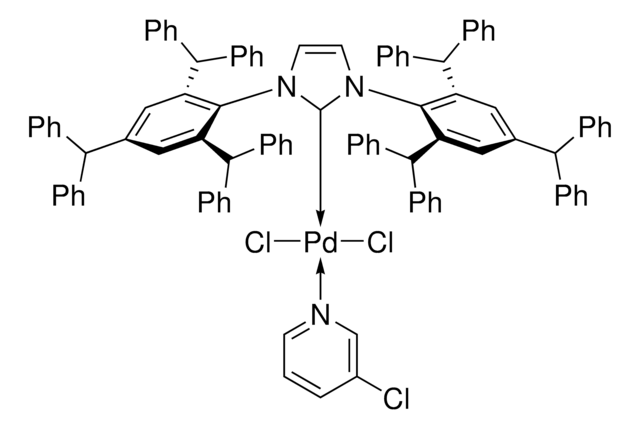
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门