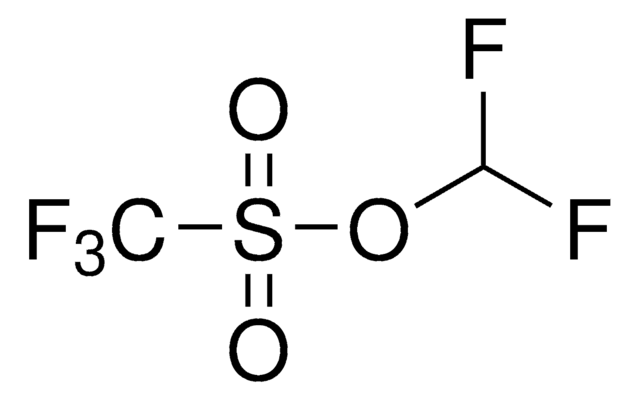
About This Item
推荐产品
品質等級
化驗
95%
形狀
solid
反應適用性
reaction type: C-C Bond Formation
reaction type: Fluorinations
reagent type: catalyst
reaction type: C-H Activation
reagent type: diversification reagent
官能基
fluoro
sulfinic acid
儲存溫度
2-8°C
SMILES 字串
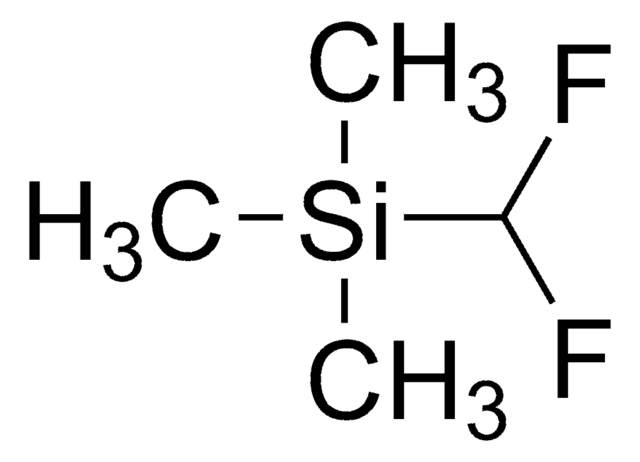
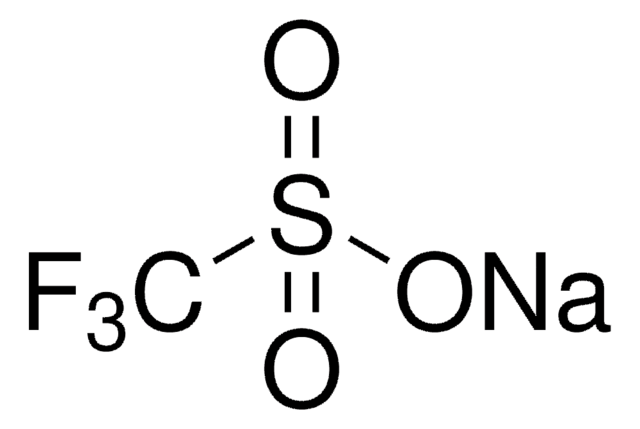
FC(F)S(=O)O[Zn]OS(=O)C(F)F
InChI
1S/2CH2F2O2S.Zn/c2*2-1(3)6(4)5;/h2*1H,(H,4,5);/q;;+2/p-2
InChI 密鑰
UGEYAPVLXKEKMP-UHFFFAOYSA-L
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
杂环化合物的实用固有碳氢功能化
DFMS 是一种新型的有机底物自由基直接二氟甲基化试剂。这种温和、操作简单、化学选择性和可放大的二氟甲基化方法与一系列不同复杂程度的含氮杂芳烃底物以及选择的共轭 p-系统和硫醇类相容。†
一种新的直接二氟甲基化试剂
在 Phil S. Baran 教授的 教授和产品门户网站 了解更多信息。
聯結
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
商品
The synthesis of heteroaromatic and aromatic compounds is at the heart of the chemical industry. The ever-growing demand for new chemical entities, coupled with dwindling resources and time constraints allotted to any given research project, a rapid way to diversify (hetero)aromatic scaffolds is needed.
The synthesis of heteroaromatic and aromatic compounds is at the heart of the chemical industry. The ever-growing demand for new chemical entities, coupled with dwindling resources and time constraints allotted to any given research project, a rapid way to diversify (hetero)aromatic scaffolds is needed.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

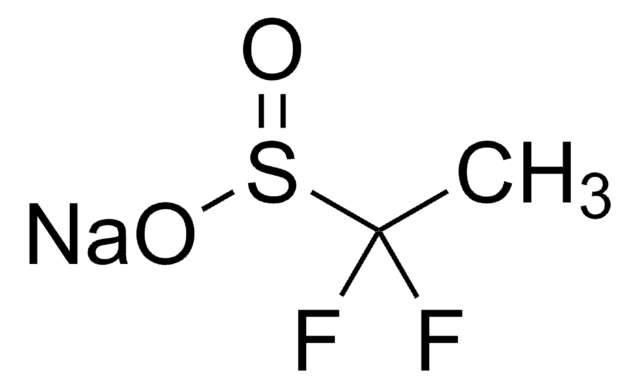
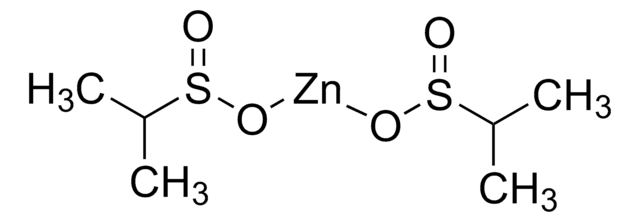
![二[双(三氟甲基磺酰)亚胺]锌 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)