推荐产品
品質等級
化驗
95%
形狀
solid
反應適用性
reaction type: C-C Bond Formation
reagent type: catalyst
reaction type: C-H Activation
reagent type: linker
官能基
azide
fluoro
sulfinic acid
儲存溫度
2-8°C
SMILES 字串
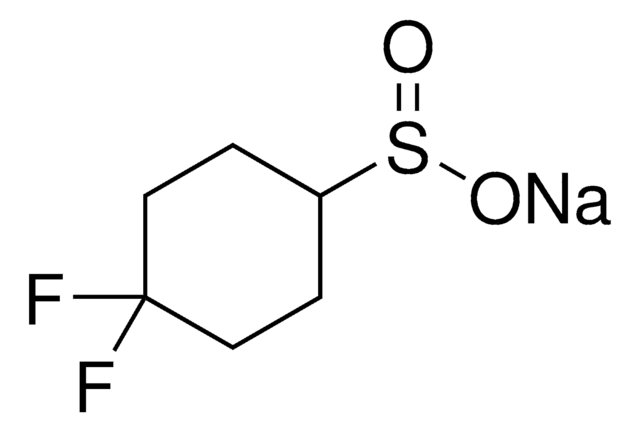
[Na+].[O-]S(=O)C(F)(F)CCCCCCN=[N+]=[N-]
InChI
1S/C7H13F2N3O2S.Na/c8-7(9,15(13)14)5-3-1-2-4-6-11-12-10;/h1-6H2,(H,13,14);/q;+1/p-1
InChI 密鑰
FFNHIEJQAMGBCZ-UHFFFAOYSA-M
相关类别
應用
Within heteroarene platforms, DAAS-Na allows for the direct introduction of a difluoroalkyl chain carrying an azide group, which is designed for the purpose of bioconjugation. Through a Huisgen cycloaddition (a "click" reaction), the azide moiety can then be reacted with a terminal alkyne that is bound to a functional molecule such as biomacromolecules. This effectively allows for a covalent linkage between a heteroarene and any target molecule, for example, between a heteroarene-containing drug and an antibody. This reagent was developed by the Baran Group and is one of several reagents for the direct alkylation of heterocycles.
Learn More at the Professor and Product Portal of Professor Phil S. Baran.
Learn More at the Professor and Product Portal of Professor Phil S. Baran.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Qianghui Zhou et al.
Journal of the American Chemical Society, 135(35), 12994-12997 (2013-08-21)
A general C-H functionalization method for the tagging of natural products and pharmaceuticals is described. An azide-containing sulfinate reagent allows the appendage of azidoalkyl chains onto heteroaromatics, the product of which can then be attached to a monoclonal antibody by
Direct synthesis of fluorinated heteroarylether bioisosteres.
Qianghui Zhou et al.
Angewandte Chemie (International ed. in English), 52(14), 3949-3952 (2013-03-06)
Fionn O'Hara et al.
Journal of the American Chemical Society, 135(32), 12122-12134 (2013-07-19)
Radical addition processes can be ideally suited for the direct functionalization of heteroaromatic bases, yet these processes are only sparsely used due to the perception of poor or unreliable control of regiochemistry. A systematic investigation of factors affecting the regiochemistry
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门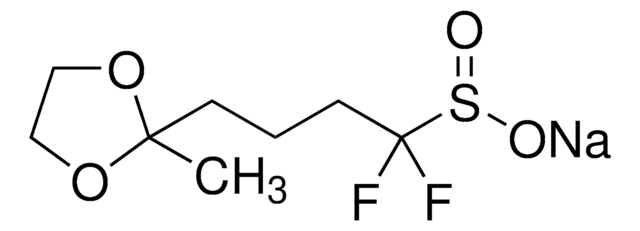

![N-[2-[2-[2-(2-叠氮乙氧基)乙氧基]乙氧基]乙基]生物素胺](/deepweb/assets/sigmaaldrich/product/structures/120/306/c9779b03-3754-4ad6-8eef-b07209e113ce/640/c9779b03-3754-4ad6-8eef-b07209e113ce.png)