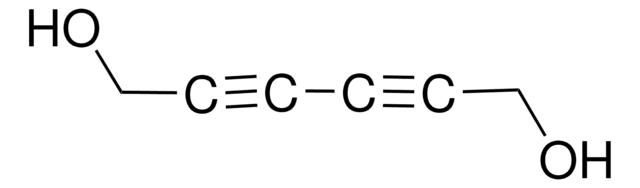推荐产品
品質等級
化驗
≥99%
bp
195 °C/733 mmHg (lit.)
密度
1.047 g/mL at 25 °C (lit.)
官能基
hydroxyl
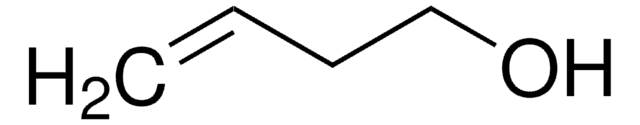
SMILES 字串
OCC(O)C=C
InChI
1S/C4H8O2/c1-2-4(6)3-5/h2,4-6H,1,3H2
InChI 密鑰
ITMIAZBRRZANGB-UHFFFAOYSA-N
一般說明
3,4-二羟基-1-丁烯,也称为3-丁烯-1,2-二醇(BDdiol),是1,3-丁二烯的代谢产物。 它形成用于合成不同手性结构单元的前体。 BDdiol经过氧化,形成羟甲基乙烯基酮(HMVK)。 在环氧化物水解酶(EH)存在下,1,2-环氧-3-丁烯(EB)水解形成BDdiol。
應用
使用3,4-二羟基-1-丁烯:
- 作为反应剂以通过连续流过程合成循环有机碳酸酯。
- 制备用于定向药物相关RNA的取代恶唑烷酮配体。
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
3-Butene-1, 2-diol: An attractive precursor for the synthesis of enantiomerically pure organic compounds.
Rao AVR, et al.
Tetrahedron, 45(22), 7031-7040 (1989)
Versatile and scalable synthesis of cyclic organic carbonates under organocatalytic continuous flow conditions
Gerardy R, et al.
Catalysis Science & Technology, 9(24), 6841-6851 (2019)
R A Kemper et al.
Chemical research in toxicology, 9(7), 1127-1134 (1996-10-01)
3-Butene-1,2-diol (BDD) is a metabolite of the carcinogenic petrochemical 1,3-butadiene. BDD is produced by cytochrome P450-mediated oxidation of 1,3-butadiene to butadiene monoxide, followed by enzymatic hydrolysis by epoxide hydrolase. The metabolic disposition of BDD is unknown. The current work characterizes
Christopher L Sprague et al.
Toxicological sciences : an official journal of the Society of Toxicology, 80(1), 3-13 (2004-05-07)
3-Butene-1,2-diol (BDD) is a major metabolite of 1,3-butadiene (BD), but the role of BDD in BD toxicity and carcinogenicity remains unclear. In this study, the acute toxicity of BDD was investigated in male Sprague-Dawley rats and B6C3F1 mice. Of the
E Malvoisin et al.
Xenobiotica; the fate of foreign compounds in biological systems, 12(2), 137-144 (1982-02-01)
1. In rat liver microsomes, 1,3-butadiene was metabolized to butadiene monoxide, which was subsequently transformed into 3-butene-1,2-diol by microsomal epoxide hydrolase. 2. In the metabolism of butadiene oxide in microsomes, four metabolites were detected, namely two stereoisomers of DL-diepoxybutane, and
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| 488216-100ML | 4061837324161 |
| 488216-1L | |
| 488216-25ML | 4061837324178 |
| 488216-500ML |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持