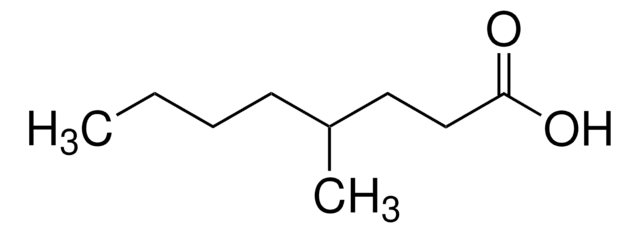
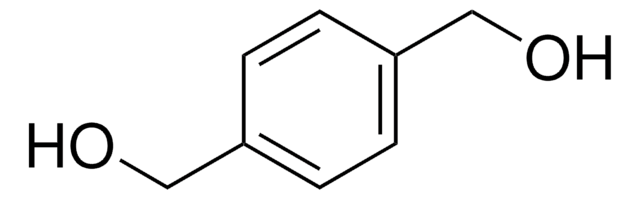
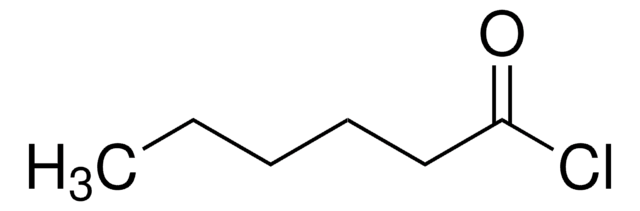
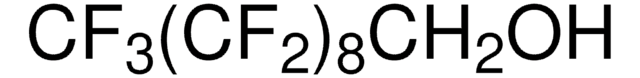推荐产品
品質等級
化驗
≥98.0% (GC)
mp
113-114 °C (lit.)
官能基
hydroxyl
儲存溫度
2-8°C
SMILES 字串
OCC#CC#CCO
InChI
1S/C6H6O2/c7-5-3-1-2-4-6-8/h7-8H,5-6H2
InChI 密鑰
JXMQYKBAZRDVTC-UHFFFAOYSA-N
一般說明
2,4-Hexadiyne-1,6-diol can be prepared from propargyl alcohol. 2,4-Hexadiyne-1,6-diol readily undergoes polymerization when heated under vacuum or inert gas atmosphere.
應用
2,4-Hexadiyne-1,6-diol may be used as a starting material in the synthesis of thiarubrine A (an antibiotic). It may also be used to synthesize disodium salt of 2,4-hexadiyne 1,6-disulfate (HDDS).
其他客户在看
Notes-use of amines in the glaser coupling reaction.
Cameron M and Bennett G.
The Journal of Organic Chemistry, 22(5), 557-558 (1957)
Self-assembled alternating multilayers built-up from diacetylene bolaamphiphiles and poly (allylamine hydrochloride): polymerization properties, structure, and morphology.
Saremi F, et al.
Langmuir, 11(4), 1068-1071 (1995)
Solid-state thermal polymerization of 2, 4-hexadiyne-1, 6-diol.
Bloor D and Stevens GC.
Journal of Polymer Science. Part B, Polymer Physics, 15(4), 703-714 (1977)
Chemistry of 1, 2-dithiins. Synthesis of the potent antibiotic thiarubrine A.
Koreeda M and Yang W.
Journal of the American Chemical Society, 116(23), 10793-10794 (1994)
The low-temperature polarized optical absorption of a crystalline diacetylene: 2, 4-hexadiyne-1, 6-diol.
Kawaoka, K.
Chemical Physics Letters, 37(3), 561-565 (1976)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门