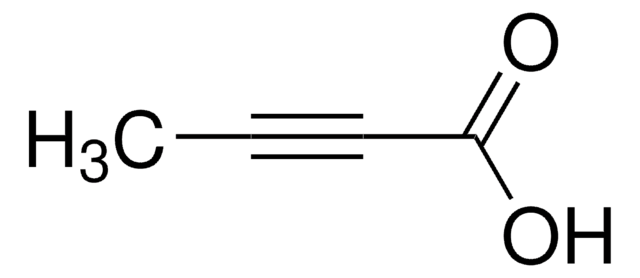所有图片(1)
About This Item
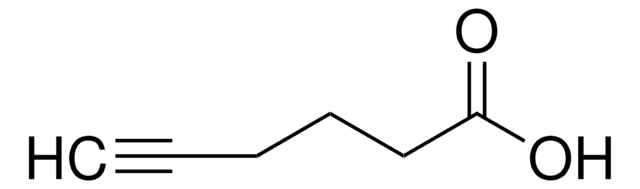
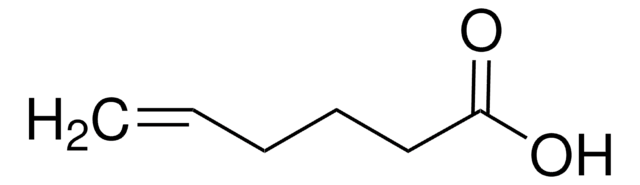
线性分子式:
HC≡C(CH2)4COOH
CAS号:
分子量:
126.15
Beilstein:
1747024
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
90%
折射率
n20/D 1.451 (lit.)
bp
93-94 °C/1 mmHg (lit.)
密度
0.997 g/mL at 25 °C (lit.)
官能基
carboxylic acid
SMILES 字串
OC(=O)CCCCC#C
InChI
1S/C7H10O2/c1-2-3-4-5-6-7(8)9/h1H,3-6H2,(H,8,9)
InChI 密鑰
OFCPMJGTZUVUSM-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
6-Heptynoic acid is an alkynoic acid with an acetylene bond. It undergoes condensation with various pyrroles to afford optical diverse fluorescent dyes with a terminal alkyne.
應用
6-Heptynoic acid may be used for the following syntheses:
- alkyne functionalized Boradiazaindacenes (BODIPY)dyes
- natural products epothilone B and D
- hymenialdisine (HMD) and aldisine (AD) affinity resins
- alkynyl esters
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
A novel catalyst with a cuboidal PdMo3S4 core for the cyclization of alkynoic acids to enol lactones.
Wakabayashi T, et al.
Angewandte Chemie (International Edition in English), 35(18), 2123-2124 (1996)
R E Taylor et al.
Organic letters, 3(14), 2221-2224 (2001-07-07)
[reaction: see text] A highly convergent total synthesis of the natural products epothilone B and D is described. The route is highlighted by efficient generation of a C12-C13 trisubstituted olefin which exploits a sequential Nozaki-Hiyama-Kishi coupling and a stereoselective thionyl
Mariano Walter Pertino et al.
Molecules (Basel, Switzerland), 19(2), 2523-2535 (2014-02-26)
Dehydroabietic acid (DHA) is a naturally occurring diterpene with different and relevant biological activities. Previous studies have shown that some DHA derivatives display antiproliferative activity. However, the reported compounds did not include triazole derivatives. Starting from DHA (8,11,13-abietatrien-18-oic acid), and
Martijn Verdoes et al.
Bioorganic & medicinal chemistry letters, 17(22), 6169-6171 (2007-09-25)
The synthesis of three acetylene functionalized BODIPY dyes is described. These dyes are used to fluorescently modify an azido functionalized epoxomicin analogue employing the Huisgen 1,3-dipolar cycloaddition, resulting in a panel of fluorescent epoxomicin derived proteasome probes.
Lauren Ray et al.
Nature communications, 7, 13609-13609 (2016-12-22)
Type I modular polyketide synthases assemble diverse bioactive natural products. Such multienzymes typically use malonyl and methylmalonyl-CoA building blocks for polyketide chain assembly. However, in several cases more exotic alkylmalonyl-CoA extender units are also known to be incorporated. In all
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门