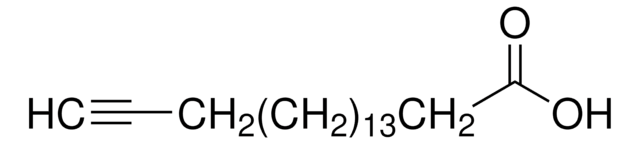推荐产品
品質等級
化驗
95%
形狀
solid
bp
110 °C/30 mmHg (lit.)
mp
54-57 °C (lit.)
官能基
carboxylic acid
儲存溫度
2-8°C
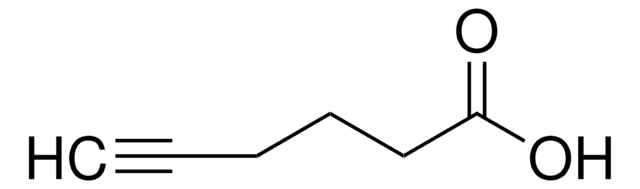
SMILES 字串
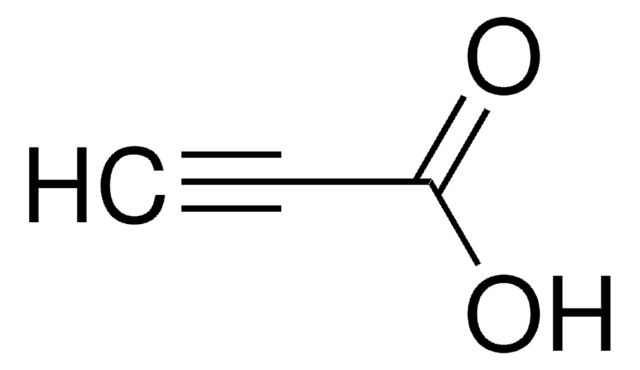
OC(=O)CCC#C
InChI
1S/C5H6O2/c1-2-3-4-5(6)7/h1H,3-4H2,(H,6,7)
InChI 密鑰
MLBYLEUJXUBIJJ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
炔丙基脲通过铜催化的分子内环化,形成烯醇内酯。在Pd催化剂存在下,它能与1-溴-1-炔烃反应,产生具有生物活性的ynenol内酯。
應用
炔丙基脲用于:
- 作为合成八个序列定义的模型寡聚体文库的结构单元
- 复杂多环杂环苯并[4,5]咪唑并[1,2-c]吡咯并[1,2-a]喹唑啉酮衍生物的一锅合成
- 合成各种allenenol内酯 [5(E)-(2-亚联烯基)-四氢-2-呋喃酮]
- 通过 双 乙炔的环合复分解反应合成细胞毒性大环内酯
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Palladium-catalyzed synthesis of new unsaturated exo-enol lactones with potential biological activity.
Bouyssi D, et al.
Tetrahedron Letters, 34(19), 3129-3130 (1993)
Xin Zhou et al.
Acta biomaterialia, 54, 128-137 (2017-03-13)
Wound healing dressings are increasingly needed clinically due to the large number of skin damage annually. Nitric oxide (NO) plays a key role in promoting wound healing, thus biomaterials with NO-releasing property receive increasing attention as ideal wound dressing. In
Thomas L Mindt et al.
The Journal of organic chemistry, 72(26), 10247-10250 (2007-11-30)
Alkynoic acids, in particular, 4-pentynoic acid derivatives, undergo intramolecular cyclizations to enol lactones under reaction conditions typically applied for the Cu(I)-catalyzed cycloaddition of terminal alkynes and azides (click chemistry). Starting from appropriate alkynoic acid derivatives, either enol lactones or 1,2,3-triazole
Tetrahedron Letters, 33, 2811-2811 (1992)
Xun Ji et al.
The Journal of organic chemistry, 78(9), 4312-4318 (2013-04-06)
An efficient and facile Au(I)/Ag(I)-catalyzed cascade method has been developed for one-pot synthesis of the complex polycyclic heterocycles benzo[4,5]imidazo[1,2-c]pyrrolo[1,2-a]quinazolinone derivatives through treatment of the substituted 2-(1H-benzo[d]imidazol-2-yl)anilines with 4-pentynoic acid or 5-hexynoic acid. The strategy features a Au(I)/Ag(I)-catalyzed one-pot cascade process
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| 232211-1G | 4061838783394 |
| 232211-250MG | |
| 232211-5G | 4061838783400 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持