推荐产品
化驗
98%
形狀
solid
mp
102-104 °C (lit.)
官能基
amine
nitro
儲存溫度
2-8°C
SMILES 字串
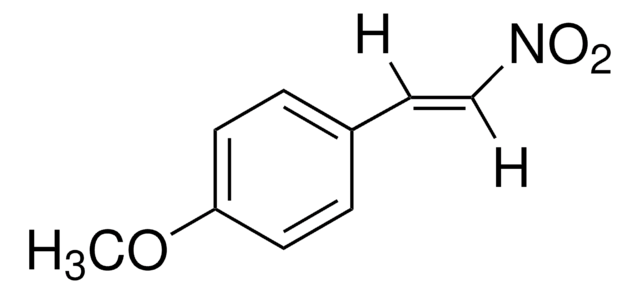
[H]\C(=C(\[H])[N+]([O-])=O)c1ccc(C)cc1
InChI
1S/C9H9NO2/c1-8-2-4-9(5-3-8)6-7-10(11)12/h2-7H,1H3/b7-6+
InChI 密鑰
JSPNBERPFLONRX-VOTSOKGWSA-N
一般說明
trans-4-Methyl-β-nitrostyrene ((E)-1-methyl-4-(2-nitrovinyl)benzene) is a nitrolefin. Its asymmetric Michael addition with benzaldehyde in the presence of silylated pyrrolidine catalyst has been reported. Its hydrogenation in the presence of Pd(II) complexes of (Z)-2-((quinolin-3-ylimino)methyl)phenol as catalyst has been studied.
應用
trans-4-Methyl-β-nitrostyrene may be used as a reagent in the synthesis of N-benzylpyrrolomorphinans and 4-oxo-2-aryl-4H-chromene-3-carboxylate derivatives.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Silylated pyrrolidines as catalysts for asymmetric Michael additions of aldehydes to nitroolefins.
Ralph Husmann et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(42), 12549-12552 (2010-09-30)
Pd (II) complexes based on quinoline derivative: Structural characterization and their role as a catalyst for hydrogenation of (E)-1-methyl-4-(2-nitrovinyl) benzene.
Azam M, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 123, 1-6 (2014)
Sanjay K Srivastava et al.
Journal of medicinal chemistry, 45(2), 537-540 (2002-01-11)
A new method for the preparation of N-benzylpyrrolomorphinans has been developed. Thus Michael reaction of the benzylimines of oxycodones and oxymorphones with nitrostyrenes gave a series of 4'-aryl-N-benzylpyrrolomorphinans. These were selective delta antagonists of much higher in vitro potency (with
Manoj R Zanwar et al.
The Journal of organic chemistry, 77(15), 6495-6504 (2012-07-20)
The unusual alcohol mediated formation of 4-oxo-2-aryl-4H-chromene-3-carboxylate (flavone-3-carboxylate) derivatives from 4-hydroxycoumarins and β-nitroalkenes in an alcoholic medium is described. The transformation occurs via the in situ formation of a Michael adduct, followed by the alkoxide ion mediated rearrangement of the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门