所有图片(1)
About This Item
线性分子式:
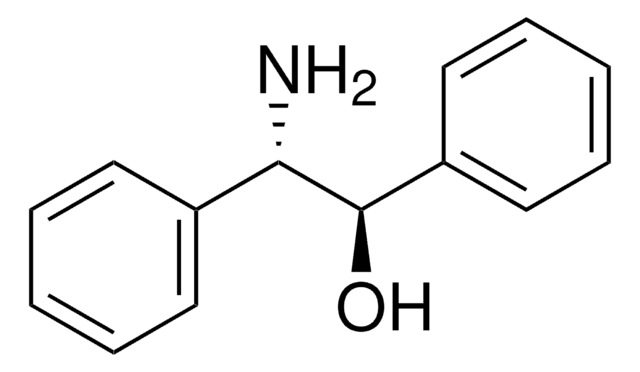
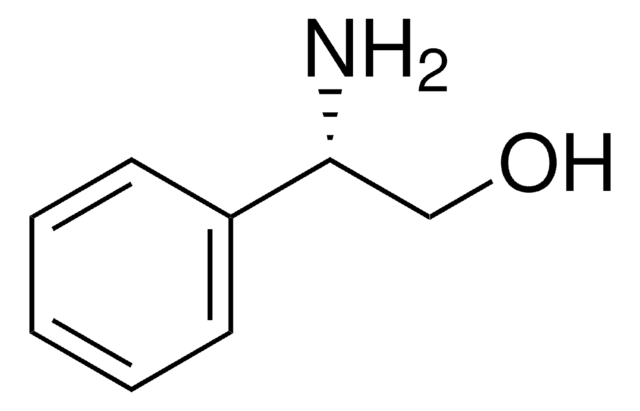
C6H5CH(NH2)CH(C6H5)OH
CAS号:
分子量:
213.28
Beilstein:
1212828
MDL號碼:
分類程式碼代碼:
12352116
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
99%
形狀
solid
光學活性
[α]25/D +7.0°, c = 0.6 in ethanol
mp
142-144 °C (lit.)
官能基
amine
hydroxyl
phenyl
SMILES 字串
N[C@@H]([C@@H](O)c1ccccc1)c2ccccc2
InChI
1S/C14H15NO/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-14,16H,15H2/t13-,14+/m1/s1
InChI 密鑰
GEJJWYZZKKKSEV-KGLIPLIRSA-N
正在寻找类似产品? 访问 产品对比指南
應用
(1S,2R)-(+)-2-Amino-1,2-diphenylethanol can be used:
- To prepare vanadium(V) Schiff base complexes, which are used as catalysts in the oxidation of sulfides and olefins.
- To prepare chiral selectors, which are immobilized on aminated silica gel, applicable as chiral stationary phase in HPLC.
- To immobilize on the frame of α-zirconium phosphate to yield layered zirconium phosphonates, which are used in the heterogeneous catalysis.
- As a chiral auxiliary in the preparation of homopropargylic alcohols from aliphatic and aromatic aldehydes.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Zirconium phosphonate immobilized chiral amino alcohol for heterogeneous enantioselective addition of diethylzinc to benzaldehyde
Zheng B, et al.
Catalysis Communications, 8(12), 1923-1928 (2007)
Vanadium (V) complexes with chiral tridentate Schiff base ligands derived from 1S, 2R (+)-2-amino-1, 2-diphenylethanol and with acetohydroxamate co-ligand: Synthesis, characterization and catalytic activity in the oxidation of prochiral sulfides and olefins
Romanowski G, et al.
J. Mol. Catal. A: Chem., 381, 148-160 (2014)
Chuan-Qi Yin et al.
Chirality, 21(4), 442-448 (2008-07-26)
A chiral selector was prepared through the reaction between (1S,2R)-(+)-2-amino-1,2-diphenylethanol and phenyl isocyanate. This selector was immobilized on aminated silica gel, respectively, with bifunctional group linkers of 1,4-phenylene diisocyanate, methylene-di-p-phenyl diisocyanate, and terephthaloyl chloride to produce corresponding three chiral stationary
Michael L Berger et al.
Bioorganic & medicinal chemistry, 17(9), 3456-3462 (2009-04-07)
We resolved 1,2-diphenylethylamine (DPEA) into its (S)- and (R)-enantiomer and used them as precursors for synthesis of (S)- and (R)-1-(1,2-diphenylethyl)piperidine, flexible homeomorphs of the NMDA channel blocker MK-801. We also describe the synthesis of the dicyclohexyl analogues of DPEA. These
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门