推荐产品
品質等級
化驗
98%
形狀
solid
光學活性
[α]20/D +52°, c = 0.6 in ethanol
mp
118-120 °C
官能基
amine
hydroxyl
phenyl
SMILES 字串
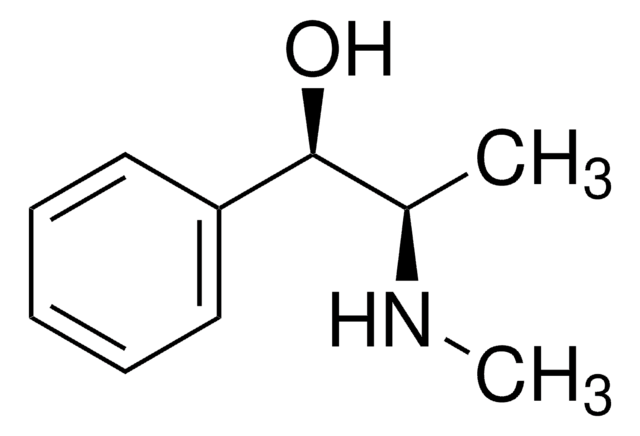
CN[C@@H](C)[C@@H](O)c1ccccc1
InChI
1S/C10H15NO/c1-8(11-2)10(12)9-6-4-3-5-7-9/h3-8,10-12H,1-2H3/t8-,10+/m0/s1
InChI 密鑰
KWGRBVOPPLSCSI-WCBMZHEXSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
(1S,2S)-(+)-Pseudoephedrine, a natural enantiomer of ephedrine, is a decongestant commonly used in cold and allergy medicines.
應用
(1S,2S)-(+)-Pseudoephedrine condenses with N,N-diisopropyl-2-formyl-1-naphthamide to form the corresponding oxazolidine derivative as a single diasterisomer.
(1S,2S)-(+)-Pseudoephedrine may be used as a chiral auxillary in asymmetric synthesis of enantioenriched organic compounds. It may also be used to prepare a novel tertiary pseudo C2-symmetric 1,2-diamine, which facilitates the enantioselective addition of methyl lithium to imines with better yield.
生化/生理作用
非选择性肾上腺素激动剂;减充血剂
應用
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
从最新的版本中选择一种:
分析证书(COA)
Lot/Batch Number
其他客户在看
James K Cunningham et al.
Drug and alcohol dependence, 126(1-2), 55-64 (2012-05-18)
Clandestine laboratory operators commonly extract ephedrine and pseudoephedrine-precursor chemicals used to synthesize methamphetamine-from over-the-counter cold/allergy/sinus products. To prevent this activity, two states, Oregon in 07/2006 and Mississippi in 07/2010, implemented regulations classifying ephedrine and pseudoephedrine as Schedule III substances, making
Beatriz Alonso et al.
The Journal of organic chemistry, 78(2), 614-627 (2012-12-25)
We have developed an efficient protocol for carrying out the stereocontrolled formal conjugate addition of hydroxycarbonyl anion equivalents to α,β-unsaturated carboxylic acid derivatives using (S,S)-(+)-pseudoephedrine as chiral auxiliary, making use of the synthetic equivalence between the heteroaryl moieties and the
(-)-Ephedrine as an auxiliary for the asymmetric synthesis of atropisomeric amides by dynamic resolution under thermodynamic control.
Clayden J and Lai LW.
Tetrahedron Letters, 42(18), 3163-3166 (2001)
A new pseudo C2-symmetric tertiary diamine for the enantioselective addition of MeLi to aromatic imines.
Gille S, et al.
Tetrahedron Asymmetry, 17(7), 1045-1047 (2006)
Pseudoephenamine: a practical chiral auxiliary for asymmetric synthesis.
Marvin R Morales et al.
Angewandte Chemie (International ed. in English), 51(19), 4568-4571 (2012-03-31)
Chromatograms
application for HPLCapplication for HPLCapplication for HPLC我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门