推荐产品
品質等級
化驗
98%
形狀
solid
光學活性
[α]20/D −51°, c = 0.6 in ethanol
mp
118-120 °C (lit.)
官能基
amine
hydroxyl
phenyl
SMILES 字串
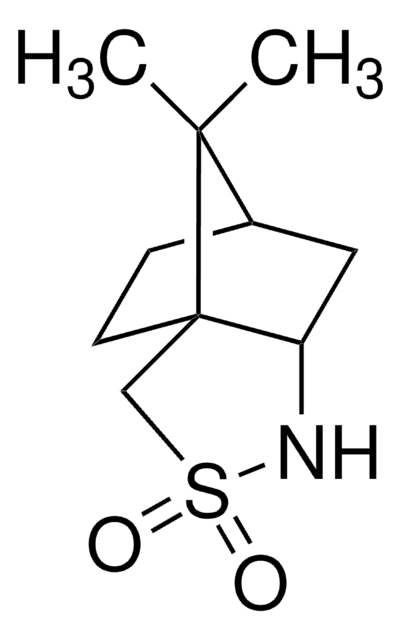
CN[C@H](C)[C@H](O)c1ccccc1
InChI
1S/C10H15NO/c1-8(11-2)10(12)9-6-4-3-5-7-9/h3-8,10-12H,1-2H3/t8-,10+/m1/s1
InChI 密鑰
KWGRBVOPPLSCSI-SCZZXKLOSA-N
一般說明
(1R,2R)-(-)-Pseudoephedrine is an enantiomer of ephedrine mainly used as a chiral auxiliary for asymmetric synthesis.
應用
(1R,2R)-(-)-Pseudoephedrine may be used in the preparation of chiral relay ligands, which can catalyze the addition of diethylzinc to aldehydes leading to the corresponding secondary alcohol. The immobilization of pseudoephedrine on Merrifield resin forms a chiral linker, which may be used for the asymmetric alkylation of amides via solid-phase approach.
應用
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1 - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Enantioselective diethylzinc additions to aldehydes catalyzed by chiral relay ligands.
Sibi MP and Levi M. Stanley.
Tetrahedron Asymmetry, 15(21), 3353-3356 (2004)
Pseudoephedrine as a practical chiral auxiliary for the synthesis of highly enantiomerically enriched carboxylic acids, alcohols, aldehydes, and ketones.
Myers AG, et al.
Journal of the American Chemical Society, 119(28), 6496-6511 (1997)
Evaluation of a pseudoephedrine linker for asymmetric alkylations on solid phase.
Hutchison PC, et al.
Organic Letters, 4(26), 4583-4585 (2002)
Chromatograms
application for HPLCapplication for HPLCapplication for HPLC我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门