298352
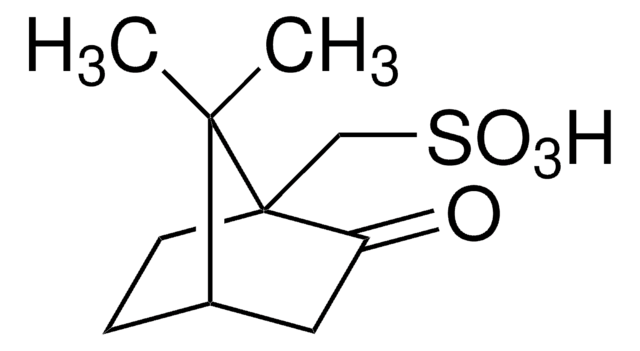
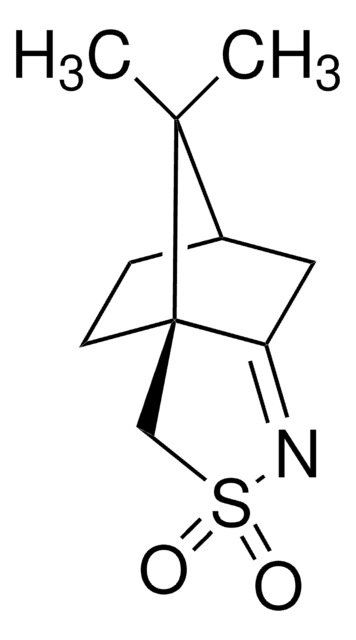
(1S)-(-)-2,10-樟脑磺内酰胺
98%
别名:
(-)-10,2-樟脑磺内酰胺, (-)-exo-10,2-莰烷磺内酰胺, (1S,5R)-10,10-二甲基-3-硫杂-4-氮杂三环[5.2.1.01,5]癸烷-3,3-二氧化物, [3aS-(3aα,6α,7aβ)]-六氢-8,8-二甲基-3H-3a,6-亚甲基-2,1-苯并异噻唑基-2,2-二氧化物
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C10H17NO2S
CAS号:
分子量:
215.31
Beilstein:
83811
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
形狀
solid
光學活性
[α]19/D −32°, c = 5 in chloroform
mp
181-183 °C (lit.)
SMILES 字串
[H][C@@]12CC[C@]3(CS(=O)(=O)N[C@@H]3C1)C2(C)C
InChI
1S/C10H17NO2S/c1-9(2)7-3-4-10(9)6-14(12,13)11-8(10)5-7/h7-8,11H,3-6H2,1-2H3/t7-,8-,10-/m1/s1
InChI 密鑰
DPJYJNYYDJOJNO-NQMVMOMDSA-N
一般說明
(1S,2R,4R)-(−)-2,10-Camphorsultam may be employed as a chiral probe for the optical resolution by HPLC and X-ray crystallographic determination of the absolute stereochemistry of carboxylic acids.
應用
(1S)-(−)-2,10-Camphorsultam may be used in the asymmetric synthesis of (S)- and (R)-N-Fmoc-S-trityl-α-methylcysteine. It may be used as proton source in the synthesis of chiral α,γ-substituted γ-butyrolactones.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
A chiral probe useful for optical resolution and X-ray crystallographic determination of the absolute stereochemistry of carboxylic acids.
Harada N, et al.
Tetrahedron Asymmetry, 4(8), 1755-1758 (1993)
M H Xu et al.
The Journal of organic chemistry, 66(11), 3953-3962 (2001-05-26)
A highly useful method for the synthesis of optically active alpha,gamma-substituted gamma-butyrolactones has been developed. The SmI(2)-induced reductive coupling of chiral 2-alkyl acrylates derived from isosorbide with ketones in the presence of (1S)-(-)-2,10-camphorsultam as a proton source give the chiral
Satendra Singh et al.
The Journal of organic chemistry, 69(13), 4551-4554 (2004-06-19)
(1R)-(+)-2,10- and (1S)-(-)-2,10-camphorsultam were acylated with ethyl 2-phenylthiazoline 4-carboxylate to afford (+)- and (-)-2-phenylthiazolinylcamphorsultam, which were stereoselectively alkylated with MeI in the presence of n-BuLi. Alkylation of these phenylthiazolinylcamphorsultams occurred from the beta-face rather than alpha-face, resulting in the formation
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门